1479
Dependence of Focused-Ultrasound Induced Blood-Brain Barrier Opening Effect with Exposure Time: Evaluation via Dynamic Contrast-Enhanced Magnetic-Resonance Imaging1Department of Diagnostic Radiology and Intervention, Chang-Gung Memorial Hospital, Taoyuan, Taiwan, 2Department of Electrical Engineering, Chang-Gung University, Taoyuan, Taiwan, 3Department of Research and Development, NaviFUS corp., Taipei, Taiwan, 4Institute of Biomedical Engineering, National Taiwan University, Taipei, Taiwan, 5Interdisciplinary Institute of Neuroscience and Technology, Zhejiang University, Hangzhou, China
Synopsis
FUS exposure with presence of microbubbles can transiently open the BBB at targeted brain tissues. The study purpose is to investigate the dependency of the BBB opening effect with ultrasound exposure time by DCE-MRI. Our result showed extending exposure time can effectively increase FUS-induced BBB opening degree without causing tissue damage. We also proposed a strategy by adjusting exposure time during the multiple exposures to overcome the effects that microbubbles concentration dynamic changed after IV bolus injection. This approach of control FUS exposure time may bring technology advances of FUS-induced BBB opening to deliver drug for CNS disease treatment.
Purpose
Focused ultrasound (FUS) exposure with intravascular injection of microbubbles can transiently open the blood-brain barrier (BBB) at targeted CNS tissue1-2. However, safety increase BBB opening scale and persist BBB opening effect during microbubble concentration decay over time in circulation are key requirements of delivery of therapeutic molecules into the brain by FUS-induced BBB opening. Our purpose is using DCE-MRI to further investigate the feasibility of increase and persist BBB opening effect by adjust FUS exposure time in single or multiple exposures.Method
20 Sprague-Dawley rats were used in this study. All rats were separated into five groups. Each Rat was under isoflurane anesthesia first. 0.5 MHz focused ultrasound transducers (diameter = 64 mm, radius of curvature = 63mm, duty cycle: 1%; PRF: 1 Hz) was used to transcranially single and multiple exposures with 0.4 MPa (exposure level was chosen to avoid brain hemorrhage3) on hemisphere of rat. Burst-tone mode ultrasound was delivered in five groups with 5 different exposure time (single exposure: 1st group (15s), 2nd group (90s), 3rd group (120s); multiple exposures: 4th group (fixed 120s), 5th group (15s, 30s, 60s, 120s,) in the presence of ultrasound microbubbles (Sonovue, Bracco). After exposure, Evans Blue (EB) was administrated intravenously and rats were conducted MRI scan (7T, ClinScan, Bruker) for 10 mins. DCE T1-weighted imaging (3D FLASH T1 sequence, TE/TR = 0.76 ms/2.31 ms; ST = 0.8 mm; FA = 5°/10°/15°/20°/25°/30°; matrix: 192 x 132) was performed to evaluate the permeability of the opened BBB. Permeability was obtained based on data post analysis using the Extended-Kety model4-6 to generate Ktrans maps. In addition, Susceptibility-weighted imaging (SWI) sequences were acquired to identify possible tissue hemorrhage associated with FUS-induced BBB opening. All animals were sacrificed 2 hours after ultrasound exposure. EB concentration quantitation7 and Hematoxylin eosin (H&E) were performed for analysis.Result
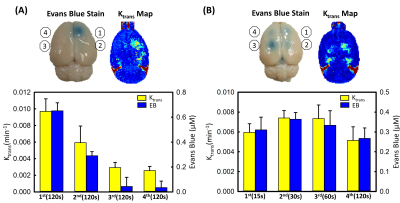
Figure 1 showed a typical comparison of the EB-stained brains and Ktrans map via DCE-MRI in single exposure with 3 different exposure time. The EB-stained brains confirmed the opening of the targeted BBB, and Ktrans which represented the BBB-opened scale linearly correlated with the FUS exposure time (r2=0.9779). Figure 2 showed the corresponding MR-SWI and HE stained examinations to confirm absence of micro-intracerebral hemorrhage in FUS exposure time at 120s under 0.4MPa. Figure 3 showed the correlations of exposure time with quantitated penetrated EB/Ktrans value under multiple FUS exposures by applying exposure-time adjusting scheme. Figure 3(A), under fixed exposure time (120s), only first two points were observed clearly BBB-opening induced by FUS in EB-stained brains and Ktrans map (Ktrans: 1st point was 0.0097 min−1 and decay to 0.0029min−1 at the 4th point; EB: 1st point was 0.65μm, and decay to 0.34μm at the 4th point). When adjusting the exposure time, both EB-stained brains and Ktrans map were observed clearly BBB-opening induced by FUS in 4 exposure points. Ktrans value and EB concentration didn’t show sharp changes across all exposure points, figure 3(B). (Ktrans: the 1st point was 0.0062 min−1 and can be maintained to 0.0054min−1 at the 4th point; EB: the 1st point was measured to be 0.31μm, whereas the 4th point still maintained to be 0.26μm; both p> 0.05). The detail values were summarized in Table 1.Discussion
High correlation between exposure time and Ktrans value in 0.62MI was observed, implying that FUS exposure time is another capable parameters to increase FUS-BBB opened scale except for increase MI. In addition, there is no erythrocyte extravasations was observed when extend exposure time to 120s which mean increase exposure time can not only increase BBB permeability but also avoid brain hemorrhage (side effect of increase MPa ). However, microbubbles concentration will decay over time to decrease BBB opening effect by FUS. But these is no significant decrease value of Ktrans or EB concentration when multiple FUS exposures with adjusted exposure time which implied geometrically increased exposure time successfully compensated for the degradation of MB concentrations in circulation over time.Conclusion
This study demonstrated the alternate method to effective increase FUS-induced BBB opening degree by extend FUS exposure time under safety MPa and avoid brain hemorrhage. However, the microbubble concentration will decay by the time in circulation. We also demonstrated the adaptive exposure time can compensate for microbubble degradation to produce a persistent BBB-opening effect by delivering multiple exposures to cover a large treatment region. This approach of control FUS exposure time may bring technology advances and facilities the clinical application of FUS-induced BBB opening to deliver therapeutic molecules for CNS disease treatment.Acknowledgements
Tis work was supported by the Ministry of Science and Technology, TAIWAN, under grants nos 104-2221-E-182-034, 104-2221-E-182-034, 105-2221-E-182-022, 105-2221-E-182-022, 105-2923-B-002-001-MY3, and byChang Gung Memorial Hospital, TAIWAN, under grants nos CIRPD2E0051-53, CMRPD2D0111-13References
1.Hynynen, K., McDannold, N., Vykhodtseva, N. & Jolesz, F. A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220, 640–646 (2001).
2. Liu, H. L., Fan, C. H., Ting, C. Y. & Yeh, C. K. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics 4, 432–444, doi: 10.7150/thno.8074 (2014).
3. P.C. Chu, W.Y. Chai, C.H. Tsai, S.T. Kang, C.K. Yeh, H.L. Liu, Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging, Sci Rep, 6 (2016) 33264.
4.P.S. Tofts, A.G. Kermode, Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts, Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 17 (1991) 357-367.
5.P.S. Tofts, Modeling tracer kinetics in dynamic Gd-DTPA MR imaging, Journal of magnetic resonance imaging : JMRI, 7 (1997) 91-101.
6.P.S. Tofts, G. Brix, D.L. Buckley, J.L. Evelhoch, E. Henderson, M.V. Knopp, H.B. Larsson, T.Y. Lee, N.A. Mayr, G.J. Parker, R.E. Port, J. Taylor, R.M. Weisskoff, Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols, Journal of magnetic resonance imaging : JMRI, 10 (1999) 223-232
7.W.Y. Chai, P.C. Chu, M.Y. Tsai, Y.C. Lin, J.J. Wang, K.C. Wei, Y.Y. Wai, H.L. Liu, Magnetic-resonance imaging for kinetic analysis of permeability changes during focused ultrasound-induced blood-brain barrier opening and brain drug delivery, Journal of controlled release : official journal of the Controlled Release Society, 192 (2014) 1-9.
Figures