1477
Accelerated imaging for visualizing interventional devices using parallel acquisition and compressed sensing reconstructionSamira Vafay Eslahi1, Caiyun Shi2, Haifeng Wang2, Yifeng Ye3, Hanwei Chen3, Guoxi Xie4, and Jim Ji1
1Electrical and Computer Engineering, Texas A&M university, College Station, TX, United States, 2Shenzhen Institutes of Advanced Technology, Lauterbur Research Center for Biomedical Imaging, Shenzhen, China, 3Department of Radiology, Panyu Central Hospital, Guangzhou, China, 4Department of Biomedical Engineering, Guangzhou Medical University, Qingyuan, China
Synopsis
Visualizing implanted and/or surgical devices is crucial for interventional radiology. Conventional MRI shows the devices as dark voids or with metal artifacts. Recent methods based on susceptibility mapping using fast spin-echo sequences can offer positive contrast visualizations, but they are relatively slow. In this work, parallel acquisition and compressed sensing reconstruction are integrated to accelerate the phase-sensitive acquisition and reconstruction. Applications in brachytherapy, biopsy and stent placement are demonstrated with simulations from real data. The proposed method can increase the acquisition speed by four while preserving the images quality.
Introduction
Due to the susceptibility artifacts, metallic devices such as brachytherapy seeds, biopsy needles or stents are difficult to visualize and localize accurately on conventional MRI images. In this study, a parallel acquisition and compressive reconstruction method are developed to shorten the scan time while providing positive-contrast visualization of the devices. The reconstruction is based on a combination of GRAPPA and compressive sensing (CS) 1-4, which preserves the phase information for susceptibility mapping. Simulated studies show the proposed method can increase the acquisition speed by a factor of 4 to 8 without compromising visualization quality.Materials and methods
The simulation and reconstruction pipeline are shown in fig. 1. The k-space undersampling is similar to the one in 4. The combined undersampling factor is termed R. Data are acquired using a modified spin echo sequence with a shifted 180-degree RF pulse 5 so that phase change due to susceptibility can be measured. During reconstruction, two acquisitions (with and without echo shift) are processed separately. For each acquisition, individual channel images are generated by first reconstructing reduced-FOV images using CS, and then reconstructing whole-FOV images using GRAPPA. Note that both steps keep the complex images and phase information for each channel. In the next step, all channel images from two acquisitions are subtracted and combined using a weighted summation. The minimization algorithm is solved by, min(||Ψfl ||1 + α||fl||TV) s.t. ||Fufl - du,l||2 ≤ ϵ, where Fu is the Fourier operator, du,l is the undersampled k-space data, fl is the aliased image of the lth channel, Ψ is the sparsifying transform, α is the controlling constant, and TV is the total variation. Then, the positive-contrast image is reconstructed using the sparsity-driven method described in 6. Three phantom studies were carried out. The first phantom contains five dummy brachytherapy seeds (titanium capsules each with 4.5 mm in length, 0.8 mm in diameter, and hemispherical shaped ends) with different spacing (L1 = 5 mm, L2 = 15 mm, and L3 = 10 mm) that are inserted in a porcine tissue. The second phantom is a biopsy needle (made of titanium and is 160.0 mm long and 2.0 mm in diameter) is constructed by filling a sealed plastic container with distilled water doped with 1.0 g l−1 copper sulfate solution. The third phantom is using a stent inserted in some gel (Ni-Ti alloy, 60mm of length × 20mm of diameter, and the wire diameter of 0.24mm). All phantoms were imaged on a 3T whole-body scanner using an 8-channel surface coil. Undersampled data are simulated by decimation according to Fig. 1 at R=4 and R=8. In all studies, 32 central ACS lines are used. Reconstructed magnitude images and positive contrast images of the devices (rendered with MIP view) are presented for comparison.Results
The results in Fig. 2 show that the proposed method has better seeds visualization quality. Reconstructions based on GRAPPA alone contains undesirable more artifacts, which are likely due to the phase errors. Compared with the full k-space reconstruction (reference), the artifact power in the positive contrast reconstructions is 35% for the proposed reconstruction with R=4, 64% for the proposed reconstruction with R=8, 54% for GRAPPA alone with R=4 and 56% for GRAPPA alone with R=8. Therefore, the proposed method yields improved reconstruction both qualitatively and quantitatively.Discussion
In all studies, magnitude images also show the metal devices, but voids are much larger than the physical size of the device, and/or with metal artifacts. In the brachytherapy seed experiment, PCI visualizes the seeds clearly with good definition, which also differentiates them from the simulated capillary, cavity or bones. This is because the seeds (and devices in general) have a higher susceptibility. Results also indicated that accelerate factors up to 8 can be used without significantly reduce the PCI quality. With the combined fast spin-echo sequence and undersampling acceleration, 3D acquisition in the brachytherapy seeds can be reduced to less than 2 minutes.Conclusion
The proposed method using parallel acquisition and compressed sensing reconstruction can yield PCI of small interventional devices by a factor up to eight without significant reduction of the image quality. This capability, when fully implemented, will enable PCI in the clinical setting for in a number of applications such as post-brachytherapy seed verification, biopsy needle guidance, and stent localization with MRI.Acknowledgements
This work was partially supported in part by National Science Foundation under award number 1606136, by Natural Science Foundation China under award number 81729003, by the Natural Science Foundation of Shenzhen under award number GHHZ20150316143320494, and by Qatar National Research Fund under award numbers NPRP6-241-2-102, NPRP8-1606-3-322, and NPRP8-293-2-124. Any opinions, findings, and conclusions or recommendations expressed in this paper are those of the author(s) and do not necessarily reflect the views of the funding agencies.References
[1] Griswold M A, Jakob P M, Heidemann R M, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic resonance in medicine. 2002; 47(6): 1202-1210. [2] Lustig M, Donoho D L, Santos J M, et al. Compressed sensing MRI. IEEE signal processing magazine. 2008; 25(2): 72-82. [3] Fischer A, Seiberlich N, Blaimer M, et al. A combination of nonconvex compressed sensing and GRAPPA (CS-GRAPPA). In Proceedings of the 17th Scientific Meeting of ISMRM, Honolulu, 2009. [4] Chang Y, King K F, Liang D, et al. Combining compressed sensing and nonlinear GRAPPA for highly accelerated parallel MRI," in Proc. Intl. Soc. Mag. Reson. Med. Victoria, Australia, 2012. [5] Shi C, Xie G, Zhang Y, et al. Susceptibility-based positive contrast imaging of MR compatible metallic devices based on modified fast spin echo sequences. Physics in Medicine & Biology. 2017; 62: 2505–2520. [6] Dong Y, Chang Z, Xie G, et al. Susceptibility‐based positive contrast MRI of brachytherapy seeds. Magnetic resonance in medicine. 2015; 74(3): 716-726.Figures
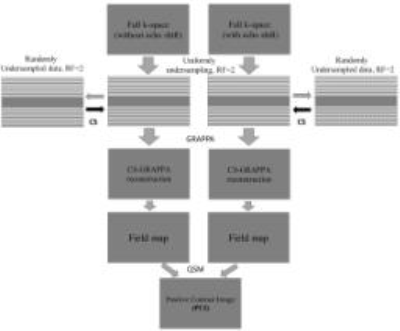
Undersampled k-space coverage and positive contrast image reconstruction procedure (with R=4). For R=8, the uniformly and randomly undersampling factors are 4 and 2, respectively. The central k-space is sampled at Nyquist intervals, and the outer k-space is first subsampled uniformly then randomly. After CS reconstruction, GRAPPA reconstructs the full FOV complex images. Based on which field maps are calculated, PCI is generated.
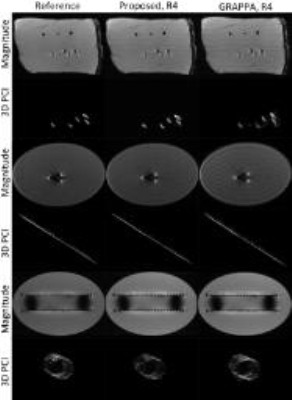
Results of the proposed method and GRAPPA
acceleration with R=4. Top two rows show the phantom images with five “dummy”
brachytherapy seeds, a plastic stick, a bamboo toothpick, and a small animal bone
inserted to simulate the human cavity, capillary, and bone, respectively. Mid rows,
show the results of the biopsy needle phantom. Bottom rows show the stent
phantom result.