Ryusuke Nakai1,2, Takashi Azuma3, Mitsuaki Toda2, Tomonobu Kodata4, and Hiroo Iwata2
1Kokoro Research Center, Kyoto University, Kyoto, Japan, 2Institute for Frontier and Medical Life Sciences, Kyoto University, Kyoto, Japan, 3Graduate School of Engineering, Kyoto University, Kyoto, Japan, 4Department of Neurosurgery, Jikei University School of Medicine, Tokyo, Japan
Synopsis
Relatively less invasive
MRI has recently been increasingly used for examination after coil embolization
of a cerebral aneurysm, but there is a risk of misdiagnosis due to magnetic
susceptibility artifacts. In this study, we developed a device composed of a
highly biocompatible alloy with magnetic susceptibility equivalent to that of mammalian
tissue, and evaluated it using both an in vitro model and rabbits. We found that
this alloy markedly reduced magnetic susceptibility artifacts and can be used
as a device in the body. We are planning to develop various implantable medical
devices using this alloy.
Purpose
Coil embolization of cerebral
aneurysms has recently been performed as a standard treatment. Periodic postoperative
examination is essential after this surgery, and relatively less invasive MRI
has recently been increasingly used for postoperative examination. However, new
problems have been discovered with increased use of MRI. Magnetic
susceptibility artifacts produced by conventional metal coils have a marked
influence on the MRI findings that determine the treatment strategy, and a risk
of misdiagnosis due to artifacts has been observed. Focusing on a diamagnetic
metal, Au, and a paramagnetic metal, Pt, with consideration for both biocompatibility
and mechanical characteristics, we investigated the composition and processing
method of an alloy prepared with these metals. Our aim was to develop an alloy
for coil treatment of cerebral aneurysm embolization with magnetic susceptibility
equivalent to that of the human tissues and thus not produce magnetic
susceptibility artifacts.Materials and Methods
Alloys
were prepared as follows: Metal pellets were placed in a zirconia crucible,
heated, melted by high frequency induction heating, and casted employing tilting
casting. The ingots were treated with hot forming to destroy solidified material.
In addition, groove rolling was applied followed by heat treatment at 1100℃ in the
atmosphere for one hour or longer. Groove rolling and heat treatment were
performed two more times. Several samples with different compositions were
prepared using this process with a single-phase treatment. In
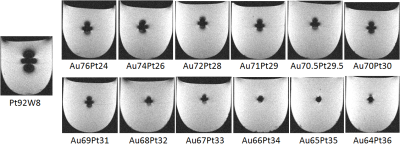
an artifact evaluation experiment, column-shaped (diameter 3 mm and length 8 mm) alloy samples were fixed with
agarose gel in a test tube and tested (Figure 1). These experimental samples and samples
prepared with the conventional PtW alloy were subjected to magnetic
susceptibility measurements using SQUID
Quantum Design MPMS-7 (7T-SQUID
Fluxmeter, Quantum Design Japan, Tokyo, Japan) and MRI using a 1.5-T scanner (MAGNETOM Sonata, Siemens A.G., Erlangen,
Germany). We
developed a coil with a small-bore diameter for high-resolution for the coil
receiving signals. MR images were acquired following the criteria of both the spin echo (TR:500ms, TE:20ms, pixel size:0.5×0.5mm, thickness:2mm, NEX:2) and gradient echo (TR:250 ms, TE:15 ms, pixel size:0.4×0.4mm, flip angle:30deg., thickness:2mm, NEX:2) sequence required by the US FDA standard artifact
evaluation test1. The artifact length from the sample margin was measured in the
acquired images using artifact measurement software prepared by us. A coil prepared with the metal composition
producing the smallest artifact and the conventional coil prepared with PtW
were placed in a rabbit aneurysm model, and MRI images were acquired employing
the 3D-TOF (TR:41ms, TE:9.21ms, pixel size:0.19×0.19mm, flip angle:25deg., thickness:0.8mm) sequence. The sizes of the artifacts in the images were compared.Results and Discussion
In the in vitro experiment,
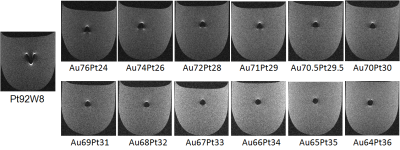
the artifact produced by the newly developed alloy was smaller than that
produced by the conventional alloy in images acquired by both the spin echo (Figure 2) and gradient echo sequence (Figure 3). The artifact length was correlated with the measured
magnetic susceptibility of the alloy, and the artifact size decreased as the
susceptibility became close to that of the surrounding tissue. Development of
an alloy producing a negligible artifact was achieved. In addition, using a
coil for aneurysm embolization prepared with this alloy material, magnetic
susceptibility artifacts produced by the aneurysm in the body could be markedly
reduced. Since small changes in the alloy preparation process may influence the
magnetic susceptibility, strict control of the preparation process is important.Conclusion
By adjusting the
composition of the embolization coil in consideration of the alloy preparation
method, an alloy with magnetic susceptibility equivalent to that of human
tissue could be prepared that markedly reduced magnetic susceptibility artifacts.
Development of MRI-compatible implantable devices for the body will be a priority.Acknowledgements
No acknowledgement found.
References
1. ASTM F2119, Annual Book of ASTM Standards, Vol. 13.01.