1462
Preliminary Experience in Off-Label Use of Ferumoxytol Contrast-enhanced Magnetic Resonance Angiography in PregnancyLindsay M Griffin1, Kim-Lien Nguyen2, Thomas M Grist1, Christopher J Francois1, Scott B Reeder3, J Paul Finn2, and Mark L Schiebler1
1Radiology, University of Wisconsin, Madison, WI, United States, 2Radiology and Medicine, University of California Los Angeles, Los Angeles, CA, United States, 3Radiology, Medical Physics, Biomedical Engineering, Medicine, and Emergency Medicine, University of Wisconsin, Madison, WI, United States
Synopsis
Recent debate about potential long-term safety of gadolinium-based contrast agents has amplified concerns about their use in pregnancy, greatly limiting options for advanced imaging in this critical patient group. We report our experience on the use of ferumoxytol contrast-enhanced magnetic resonance angiography (MRA) during pregnancy. We identified eight pregnant subjects, at two institutions, with contrast-enhanced MRI/MRA using ferumoxytol. There was one mild possible adverse event during contrast administration. There were no premature deliveries (< 35 weeks) or birth defects in five babies with available postpartum data. While preliminary, ferumoxytol holds promise as a versatile MR contrast agent in pregnancy.
Introduction
Ferumoxytol was approved in 2009 for treatment of iron deficiency anemia in adults with chronic kidney disease1 and is used off-label at several institutions to treat iron deficiency anemia during pregnancy.2,3 This agent has emerged as an off-label magnetic resonance (MR) contrast agent with a prolonged blood pool phase (plasma half-life of 14-21 hours).4 In 2015, based on post-marketing data for therapeutic use, the United States Food and Drug Administration (U.S. FDA) labeled ferumoxytol with a “black box” warning for serious and potentially fatal allergic reactions.5 However, the estimated pooled aggregate anaphylaxis rate is 0.03%.6 When used for contrast enhanced magnetic resonance imaging/angiography (CE-MRI/MRA), anaphylactoid reactions are quite rare.6 Currently, no gadolinium-based MRI contrast agents are U.S. FDA-approved for use during pregnancy due to the established long-term risk from in-utero gadolinium exposure.7 Yet, several maternal conditions occurring during pregnancy may be life-threatening to the mother and the fetus. For example, confident evaluation using non-ionizing radiation imaging modalities for conditions such as pulmonary embolism (PE) or abnormal placentation or a combination thereof remain a diagnostic challenge. Although the safety of ferumoxytol as an MRI contrast agent in pregnancy has not been established, the use of ferumoxytol as an MR contrast agent may provide a ready solution since ferumoxytol is already being used in this patient population. Therefore, the purpose of this work was to describe our experience with pregnant patients administered ferumoxytol for CE-MRI/MRA.Methods
This retrospective observational study was performed with IRB approval including a waiver of informed consent for case review. Patients were identified through a ferumoxytol safety database8 and a search of the medical record for patients with the terms related to “ferumoxytol” and “MRI”. Charts were then reviewed to confirm pregnancy at the time of the scan. Gestational age at time of study, study indication, ferumoxytol dosing, adverse events during administration, study impression, and pregnancy outcomes were collected.Results
Eight studies during pregnancy were identified (Figure 1): infection (n=1), pulmonary embolism (n=3), and placenta accreta (n=4). The gestational age at time of scan ranged from 8-34 weeks. Maternal age ranged from 21-58 years (median 30 years). Weight-based dosing of ferumoxytol was used in all cases. In pulmonary MRA, dosing was 3 mg/kg (total dose range 174-270 mg). In all other studies, dosing was 4 mg/kg (total dose range 276-363 mg). Ferumoxytol was diluted with 30-50 mL normal saline. In 5 patients, images were acquired during the first pass and steady state phases following bolus injection and in 3 patients in the steady state only, following a 15-minute infusion with the patient in the supine position. There was one possible mild adverse event involving a 30-year-old patient with a history of hypotension at 8 weeks gestation. She had transient hypotension and was treated with fluids and lower extremity elevation. Her blood pressure recovered to normal within five minutes. The indication for the PE study was listed as “episodes of hypotension”; hence, it is unclear if the hypotension was related to ferumoxytol administration. A 58-year-old patient at 29 weeks gestation was imaged for placenta accreta had hypoglycemia prior to ferumoxytol injection, recovered after treatment with D5, and underwent ferumoxytol-enhanced MRI without further issues. All studies were technically adequate. Placenta accreta was identified in one patient, was low probability in 2 patients, and too early to assess in 1 patient. Pulmonary MRA was negative for PE in all 3 patients and diagnostic to the subsegmental level (Figure 2). The patient with a renal transplant who underwent ferumoxytol-enhanced MR to exclude renal abscess had no infection or transplant abnormality (Figure 3). Fetal follow up data was available for 5/8. There were no neonatal abnormalities. No premature deliveries (<35 weeks) occurred.Discussion
There were no maternal complications, early delivery (<35 weeks), or neonatal abnormalities associated with the use of ferumoxytol-enhanced MR during pregnancy. There was one possible ferumoxytol-related mild adverse event requiring brief supportive therapy. While preliminary, in the setting of appropriate monitoring and clear beneficial indications, ferumoxytol is well tolerated as an MR contrast agent in pregnancy. Ferumoxytol-enhanced pulmonary MRA and evaluation of the placenta are examples of applications which can produce information not otherwise available. In particular, ferumoxytol-enhanced pulmonary MRA may be useful as a non-ionizing imaging test in the work up of PE during pregnancy. Future investigation in this critical area will require international collaboration through enrollment of new subjects in a registry that will be freely accessible for longitudinal follow up after the use of ferumoxytol during pregnancy.Acknowledgements
No acknowledgement found.References
- FDA prescribing information for Ferumoxytol. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022180lbl.pdf. Accessed August 8, 2017.
- Chandra I, Sun L. Iron status and choice of iron therapy during pregnancy: advantages and disadvantages. Int J Reprod Contracept Obstet Gynecol. 2015;4(5):1264-1271.
- Achebe MM et al. Blood. 2017 Feb 23;129(8):940-949. doi: 10.1182/blood-2016-08-672246. Epub 2016 Dec 29.
- Vasanawala SS et al. Safety and Technique of Ferumoxytol Administration for MRI. Magn Reson Med. 2016;75(5);2107-2111.
- US Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). https://www.fda.gov/Drugs/DrugSafety/ucm440138.htm. March 30, 2015. Accessed August 8, 2017.
- Toth GB et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017; 92:47-66.
- Ray JG et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016; 316(9):952-61.
- Nguyen KL et al. MRI with ferumoxytol: A single center experience of safety across the age spectrum. J Magn Reson Imaging. 2017;45:804-812.
Figures
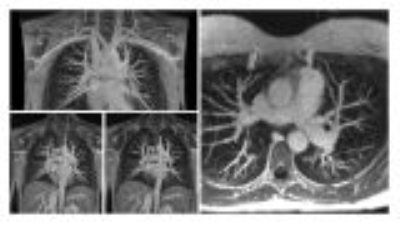
Figure 2. 30-year-old pregnant female who is 14 weeks
pregnant presenting with shortness of breath and tachycardia. Coronal and axial
maximum intensity projection images from pulmonary MR angiography. No filling defect is
identified in the pulmonary arteries to the subsegmental level. No additional findings in the chest.
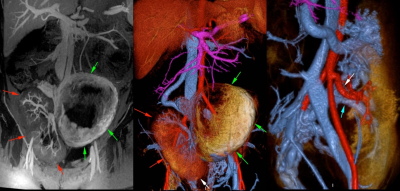
Figure 3. 21-year-old pregnant female with a
right pelvic renal transplant (red arrows).
MRA with ferumoxytol showed normal vascular anatomy in the renal
transplant (arrows in right image) and normal utero-placental enhancement
(green arrows in left and middle images).
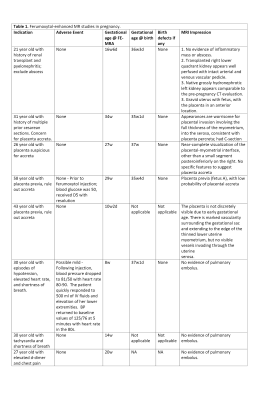
Indications and outcomes of ferumoxytol contrast enhanced magnetic resonance studies (FE-MRA) in pregnancy. w, weeks; d, days.