1455
MRI compatible neural electrodes for simultaneous deep brain stimulation and fMRI mapping1Department of Biomedical Engineering, College of Engineering, Peking University, Beijing, China, 2Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China, 3Center for Nanochemistry, Beijing Science and Engineering Center for Nanocarbons, Peking University, Beijing, China, 4Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China
Synopsis
Functional magnetic resonance imaging (fMRI) under deep brain stimulation (DBS) provides important insights into understanding the connection of the neural networks. However, such research has been limited by incompatibility of common electrode in the MR environment. To address such issue, we fabricated a novel graphene based neural microelectrode, which exhibited excellent charge storage capacity and MRI compatibility. Using such microelectrode, we successfully demonstrate deep brain stimulation of subthalamic nucleus (STN) evoked robust BOLD activation in cortex and basal ganglia nucleus of the Parkinsonian rats with minimal image artifact. Therefore, MR-compatible graphene microelectrode could provide unique opportunity for simultaneous DBS-fMRI studies.
Purpose
Fabricated the MR-compatible graphene fiber based stimulation microelectrodes with excellent electrochemical properties. Further applied the graphene fiber microelectrodes in deep brain stimulation (DBS)-fMRI to study the global activation pattern of the brain.Introduction
Functional magnetic resonance imaging (fMRI) combined with electrical stimulation provides a picture of the global spatial activation pattern of the brain. Conventional metal implantable microelectrodes (platinum−iridium alloy, tungsten, gold, nichrome, stainless steel etc.) may induce severe field distortions, thus producing image artifacts or blind spots around the microelectrodes in MRI that cause inconvenience or interference for anatomical and functional MRI studies.1,2 To give a precise and full visualization of the activation pattern using fMRI, neural microelectrodes with minimal image artifact under high field is necessary. The difference of magnetic susceptibility between the implants and surrounding tissues is usually the dominant cause for the image artifacts in MRI.3 Graphene fiber, which has a magnetic susceptibility closer to water/tissue than most metal is thought to produce less image artifacts under MRI scan.4,5 In this work, we fabricated graphene based fiber microelectrodes which showed excellent electrochemical performance and MRI compatibility. We applied this novel MR-compatible microelectrode in deep electrical stimulation of subthalamic nucleus (STN) and mapped the resulting activation pattern using 9.4 T MRI.Method
Graphene fiber was fabricated though one-step dimension-confined hydrothermal methods from suspensions of graphene oxide, which was obtained by modified Hummers’ method. DBS microelectrode was fabricated using graphene fiber with diameter of 75 μm and length of 8-10 mm. The impedance spectrum of graphene fiber microelectrodes was measured with a CHI660 electrochemical workstation. For charge injection limit measurement, a stimulator (AM systems) was used to generate constant-current rectangular pulses with increasing amplitude and apply the pulses onto graphene fiber microelectrodes immersed in 1x PBS solution. An oscilloscope was used to record the potential change of the electrodes. For electrode implantation, all rats were anesthetized using ~2% isoflurane. Graphene fiber microelectrode was implanted unilaterally into the STN. (AP: -3.8mm, ML: 2.4mm, DV: 8.2mm). A platinum−iridium(Pt-Ir) electrode with the same diameter was implanted into the STN of another rat. All MRI experiments were performed in a Bruker 9.4 Tesla and a single-loop surface coil. T2-weighted anatomical images using a RARE sequence (TR/TE= 2500/33 ms, FOV= 3 × 3 cm, slice thickness = 0.8 mm, matrix = 256 × 256). fMRI scans using 4-shot gradient echo EPI sequence (TR/TE =500/13 ms, 105 repetitions, FOV = 3 × 3 cm, matrix =80 × 80, 14 slices, slice thickness 0.8 mm). Evoked fMRI experiments were performed in 210 s, during which stimulation was applied in a 60s-OFF/30s-ON/120s-OFF cycles. DBS frequencies of 130 Hz was applied with a bipolar square-wave current of 300µA.Result and Discussion
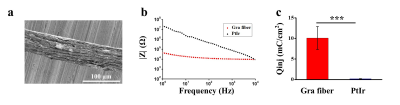
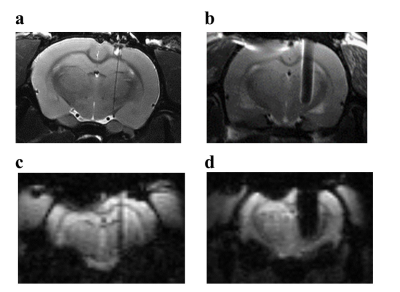
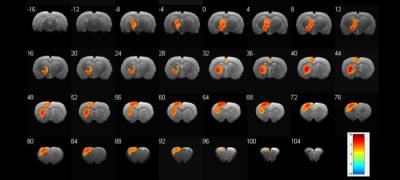
A typical scanning electron microscopy image of the graphene fiber (Fig. 1a) shows a porous structure which consists of graphene sheets aligned along the axis direction and densely stacked. Minimization of impedance and maximization of charge injection limit for neural microelectrodes are generally considered desirable to achieve safety and efficacy of stimulation. The graphene fiber microelectrodes displayed impedance that is ~8 times lower than the Pt-Ir electrodes with same diameter of 75 μm (13.8 ± 1.8 kΩ vs. 117 ± 45 kΩ at 1 kHz, Fig. 1b). The charge injection limit (Fig. 1c) of the graphene fiber microelectrodes is ~50 times larger than the Pt-Ir electrodes. This improved electrochemical performance of the graphene fiber microelectrodes arises from the porous microstructure which leads to higher electrochemical surface area (ESA)/geometric surface area (GSA) ratio. MRI images of T2 weighted RARE sequence of the rat brain with implanted microelectrodes show much larger artifact of Pt-Ir (~1.6 mm) than graphene fiber (~160 μm), both with a real diameter of 150 μm (Fig. 2a,b). MRI images of T2* weighted EPI sequences give same results (Fig. 2c, d). The negligible artifact of graphene fiber microelectrodes shows advantages in identifying the activated brain region under deep brain stimulation. As shown in Fig. 3, the electrical stimulation at the subthalamic nucleus (STN) produced significant positive BOLD responses in basal ganglion region and cortex, including motor cortex, somatosensory cortex and cingulate cortex. The activation in STN, internal and external globus pallidus, substantia nigra and thalamus nuclei was also observed, which was not visible with stimulation by Pt-Ir or other metal electrodes because of the large artifact. These results demonstrated the advantages of using MRI compatible graphene fiber microelectrodes to map the connectivity of neural circuits in brain under simultaneous DBS and fMRI.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21422301, 21373013), the National Basic Research Program of China (No. 2014CB932500, 2016YFA0200103), and China’s 1000 Young Talent Award program.References
1 Arantes PR, Cardoso EF, Barreiros MA, et al. Performing functional magnetic resonance imaging in patients with Parkinson's disease treated with deep brain stimulation. Movement Disord. 2006;21(8):1154-1162.
2 Lai HY, Younce JR, Albaugh DL, et al. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage. 2014;84(1):11-18.
3 Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys.1996;23(6):815-850.
4 Novoselov KS, Geim AK, Morozov SV, et al. Electric Field Effect in Atomically Thin Carbon Films. Science. 2004;306(5696):666-669.
5 Zhao S, Liu X, Xu Z, et al. Graphene Encapsulated Copper Microwires as Highly MRI Compatible Neural Electrodes. Nano Lett. 2016;16(12):7731-7738.
Figures