1439
Towards Whole-Skeleton Fat Fraction Mapping: The Impact of Parallel Imaging1Royal Free Hospital, London, United Kingdom, 2Centre for Medical Imaging, University College London, London, United Kingdom, 3Department of Medical Physics, University College London Hospitals, London, United Kingdom
Synopsis
Whole body MRI (WB-MRI) is increasingly used to image the skeleton in haematological diseases such as multiple myeloma (MM) and inflammatory disorders such as spondyloarthritis. WB-MRI can be used to acquire fat fraction (FF) maps, which can assess disease severity and treatment response. However, patients with bone pain find it difficult to lie in the scanner for long periods, necessitating the use of parallel imaging to accelerate the acquisition. The aim of this study was to determine the extent to which parallel imaging causes noise artifacts and fat-water swaps in FF maps, and to assess their impact on FF measurements.
Purpose
Whole body MRI (WB-MRI) is increasingly used to image the skeleton in haematological diseases such as multiple myeloma (MM) and inflammatory disorders such as spondyloarthritis. Chemical-shift encoded MRI (CSE-MRI) [1] can be used to acquire fat fraction (FF) maps of the whole body, and can assess disease burden and treatment response [2,3]. However, patients with MM and spondyloarthritis often suffer from bone pain, making it difficult for them to lie still in the scanner for extended periods and necessitating the use of parallel imaging techniques [4]. Although generally effective, parallel imaging can cause non-uniform noise artifacts [4,5] and may contribute to fat-water swaps. At present, the impact of parallel imaging on FF quantitation is poorly defined, and the choice of parallel imaging factors is often made on an empirical basis when developing WB-MRI protocols. The aim of this study was to assess the impact of Sensitivity Encoding (SENSE) on (i) artifact severity and (ii) FF measurements in the bone marrow.Methods
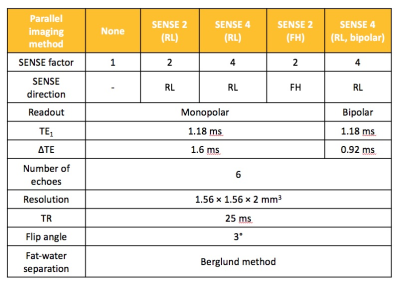
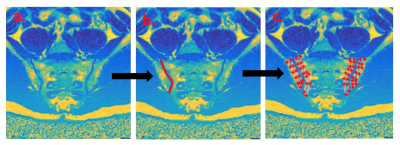
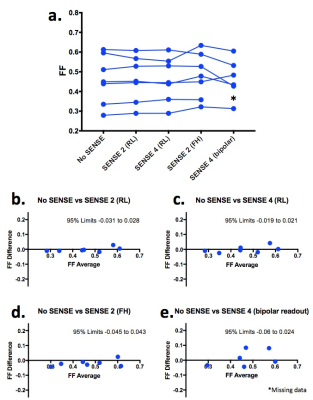
Seven healthy subjects aged 25-35yrs underwent CSE-MRI of the pelvis on a 3.0T system (Ingenia, Philips, Amsterdam, Netherlands) with anterior surface and integrated posterior coils. Subjects were scanned using a six-echo spoiled gradient echo acquisition (first echo 1.2ms, echo spacing 1.6ms, repetition time 25ms, flip angle 3°), which was repeated with various Sensitivity Encoding (SENSE) factors applied in both right-left (RL) and foot-head (FH) directions, as shown in Figure 1. The SENSE 4 acquisition was also repeated using bipolar rather than monopolar readout gradients, which enabled a shorter echo spacing and therefore an effectively higher spectral resolution [6]. For each acquisition, complex data were processed offline using an analytical three-point method previously described by Berglund et al., to generate fat fraction (FF) maps for subsequent analysis [7]. To assess the severity of fat-water swaps and non-uniform noise artifacts resulting from the use of parallel imaging, images were randomised and assessed by a radiology resident (TB) with expertise in musculoskeletal MRI and in CSE-MRI, who was blinded to acquisition parameters. Both fat-water swaps and non-uniform noise artifacts were assessed on a four point scale (0–no artefact, 1–diagnostically irrelevant, 2–diagnostically relevant, 3–non-diagnostic) [7]. Artifact severity was compared across SENSE factors using a Friedman test and Dunn’s multiple comparison test. To assess the effect of parallel imaging on FF measurements, regions-of-interest (ROIs) were placed on pelvic bone marrow in a standardised fashion using a dedicated in-house tool, which propagates ‘bands’ of subchondral bone adjacent to the sacroiliac joint after the observer defines the joint itself (Figure 2). Mean FF values for each subject were then compared between groups using a repeated measures analysis of variance (ANOVA). Additionally, Bland-Altman limits of agreement plots were used to assess agreement between individual pairs of SENSE factors.Results and Discussion
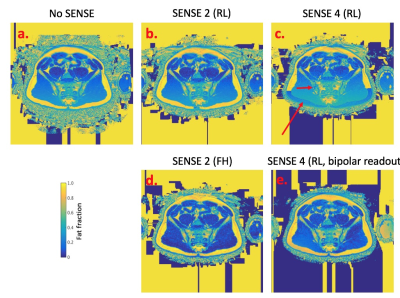
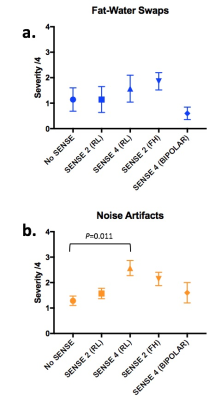
Examples of images obtained from the same subject but with varying SENSE factor are shown in Figure 3, and artifact severity scores are shown in Figure 4. The impact of SENSE factor on FF measurements is shown in Figure 5a, and individual comparisons between acquisition pairs (e.g. no SENSE vs SENSE 2) are shown in Figures 5b-e. As shown in Figures 3 and 4, increasing SENSE factors generally resulted in more severe non-uniform noise artifacts and fat-water swaps. For example, noise artifacts were significantly increased in the SENSE 4 acquisition (p=0.011) compared to the acquisition without SENSE. However, when FF measurements were compared across SENSE factors (Figure 5), the differences between pairs of measurements were generally much smaller than the means (5b-e), and also smaller than the effect of disease [3]. This suggests that the quantitative impact of increasing SENSE factor is likely to be relatively insignificant for most clinical purposes. Interestingly, at a SENSE factor of 4, fat-water swaps were less severe when using bipolar readout gradients than when using monopolar readout gradients, suggesting that the use of bipolar readouts may enable the use of higher SENSE factors whilst still keeping fat-water swaps to a minimum.Conclusions
Our data demonstrated that increasing SENSE factors resulted in more severe noise and fat-water swaps in FF maps. However, the effect of these artifacts on FF measurements was relatively small compared to the effect of disease [3,8]. Clearly, there is a balance to be struck between speed and the accuracy of FF measurements; our data will help to make rational choices regarding the use of parallel imaging in future studies involving FF quantitation. On a practical note, the use of bipolar readout gradients may enable the use of higher SENSE factors without increasing the severity of fat-water swaps.Acknowledgements
This work was undertaken at UCLH/UCL, which receives funding from the Department of Health’s NIHR Biomedical Research Centre funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
TJPB is funded by Arthritis Research UK Grant 21369.
We acknowledge the use of the ISMRM Fat-Water Toolbox (http://ismrm.org/workshops/FatWater/data.htm) for some of the results shown in this article.
References
1. Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–94.
2. Latifoltojar, A, Hall-Craggs, M, Bainbridge, A, Yong, K, Dikaios, Nikolaos, Sokolska, M, Rabin, N, Antonelli, M, Ourselin, S, Popat, R, Rismani, A, D’Sa, S, Taylor, S, Halligan, S, Punwani S. Whole-body MRI quantitative biomarkers predict response in patients with newly diagnosed symptomatic multiple myeloma following Bortezomib induction. Eur Radiol. 2017;
3. Bray, TJP, Bainbridge, A, Punwani, S, Ioannou, Y, Hall-Craggs M. Simultaneous Quantification of Bone Edema/Adiposity and Structure in Inflamed Bone Using Chemical Shift-Encoded MRI in Spondyloarthritis. Magn Reson Med. 2017; doi:10.1002/mrm.26729.
4. Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magnetic Reson Imaging. 2012;36:55–72.
5. Aja-Fernández S, Vegas-Sánchez-Ferrero G, Tristán-Vega A. Noise estimation in parallel MRI: GRAPPA and SENSE. Magn Reson Imaging. 2014;32(3):281–90.
6. Hu HH, Börnert P, Hernando D, Kellman P, Ma J, Reeder S, et al. ISMRM workshop on fat-water separation: Insights, applications and progress in MRI. Magn Reson Med. 2012;68(2):378–88.
7. Berglund J, Johansson L, Ahlström H, Kullberg J. Three-point Dixon method enables whole-body water and fat imaging of obese subjects. Magn Reson Med. 2010;63(6):1659–68.
8. Latifoltojar, A, Hall-Craggs, M, Rabin, N, Popat, R, Bainbridge, A, Dikaios, N, Sokolska, M, Rismani, A, D’Sa, S, Punwani, S, Yong K. Whole body magnetic resonance imaging in newly diagnosed multiple myeloma: early changes in lesional signal fat fraction predict disease response. Br J Haematol. 2017;176(2): 222–233.
Figures