1432
Analysis of collagen fibrillogenesis of a caprine patella tendon with magic angle imaging1Medicine, Surgery and Cancer, Imperial College London, London, United Kingdom, 2University of Adelaide, Adelaide, Australia, 3Mechanical Engineering, Imperial College London, London, United Kingdom
Synopsis
It is known that our collagen fiber alignment changes as we develop, reach maturity and then age: the crosslinking of collagen is considered one of the best biomarkers of aging. This study used magic angle imaging to visualise the collagen fiber changes between development and skeletal maturity in caprine knees. Immature tendons are less aligned during development, becoming more aligned as skeletal maturity is reached. This method has great potential to non-invasively improve our understanding of the development and degeneration of collagen rich structures.
Introduction
Controversy remains about the length and continuity of collagen fibrils in skeletally mature tendons and ligaments1. To date, it has not been possible to non-invasively measure the changes to collagen fibrils during aging or repair2. Tendon and ligaments are normally not visible on Magnetic Resonance Imaging (MRI) appearing black or gray compared to the brighter surrounding soft tissue. However, when the collagen fibers within the tendons and ligaments are orientated at the magic angle of 54.7˚ to the main magnetic field B0 they become visible. A study to assess inter-subject, intra-group variability with magic angle imaging of five caprine knees is presented.Hypothesis
All caprine knees demonstrate a highly aligned collagen fibre orientation along the length of the patella tendon.Methods
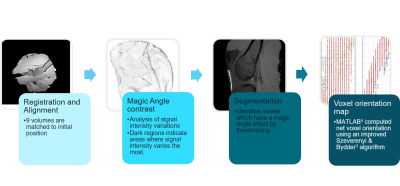
A Siemens 3T Verio (Magnetom, Erlangen) with a 12 channel head coil fitted with a specially designed holder for the test sphere containing the embedded caprine knees was scanned in 9 positions to the main magnetic field (B0). An isotropic 3D T1 FLASH sequence (TR13ms, TE4.9ms, FOV256mm, BW230Hz) was performed in each position after the test sphere was rotated. The experiment was repeated on five knees. The raw 3D T1 FLASH volumes were registered and aligned then compared to identify large variations of signal intensity. Segmentation using a thresholding technique identified voxels containing collagen. For each collagen-rich voxel the orientation vector was computed using Szeverenyi and Bydder’s3 method. Each orientation vector reflects the net effect of all the fibers comprised within a voxel. The assembly of all unit vectors represents the fiber orientation map. All steps are shown in figure 1. Signal intensities variations were measured from the central midline slice of the 3D T1FLASH images using Fiji for ImageJ4.Results
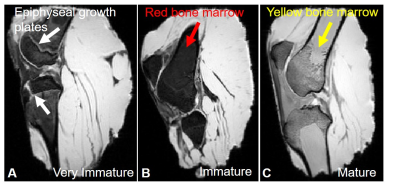
Of the five caprine knees scanned, three had mean bone marrow signal intensities of 61±3 and two had mean bone marrow signal intensities of 282±6 (Figure 2). Hypointense haemopoetic red bone marrow (B) in the immature caprine knees and hyperintense yellow marrow (C) in the older more skeletally mature caprine knees can be seen. Unfused epiphyseal plates (A) were noted in one of the caprine knees suggesting it was less than 3 months of age.
The Matlab (The MathWorks Inc., Natick, MA, USA) output for the net voxel vector collagen orientations were computed for an immature and mature patella tendon and shown in Figure 3. Each vector is coloured depending on its direction to assist in visualising the collagen orientations. The mature specimen is much more aligned and organised when compared to the immature specimen.
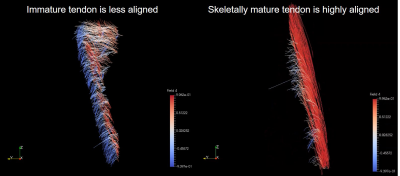
A ParaView5 streamline visualisation in 3-dimensions of the patella tendon collagen tracts of a mature and immature caprine stifle are shown in Figure 4. The immature specimen has less aligned collagen fiber tracts whereas the skeletally mature specimen is highly aligned.
Discussion
The expected outcome of highly aligned patella tendons was only demonstrated in skeletally mature caprine specimens. It was understood from the meat supplier that the caprine specimens would be aged around 12 months (±6months). The specimens were aged by the change in marrow signal demonstrated on the T1 FLASH sequence. The five caprine knees studied ranged in age from less than 3 months (femoral and tibial growth plates, red bone marrow) to more than 3 years (skeletal maturity, yellow bone marrow). A hypointensity was demonstrated by red marrow that contains approximately 40% fat, 40% water and 20% protein6. A hyperintensity was seen with yellow marrow that contains approximately 80% fat, 15% water and 5% protein6. The mean collagen fibril length increases from birth to maturity7; fibrils in mature ligaments and tendons are known to be either continuous or functionally continuous1.Conclusion
The study demonstrates the first visualisation of the collagen fibrillogenesis in caprine patella tendon using magic angle imaging. It is now possible to use MRI to improve our understanding of the development and degeneration of collagen rich structures.Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Invention for Innovation (i4i) under Grant II-LA-1111-20005. We are grateful to Charing Cross Hospital MRI department and Imaging Committee for the kind use of the Siemens 3T Verio.References
1 Provenzano, P.P., & Vanderby Jr, R., 2005. Collagen fibril morphology and organisation: Implications for force transmission in ligaments and tendon. Matrix Biology. 25:71-84.
2 Barros, E.M.K.P., Rodrigues, C.J., Rodrigues, N.R., Oliverira R.P., Barros, T.E.P., Rodrigues Jr, A.J., 2002 Aging of the elastic and collagen fibers in the human cervical interspinous ligaments. The Spine Journal. 2(1):57-62
3 Szeverenyi, N.M. & Bydder, G.M. 2011. Dipolar anisotropy fiber imaging in a goat knee meniscus. Magnetic Resonance in Medicine.65:463–470.
4 Schindelin, J.; Arganda-Carreras, I. & Frise, E. et al. (2012), "Fiji: an open-source platform for biological-image analysis", Nature methods 9(7): 676-682
5 Ahrens, J., Geveci, B. & Law, C. 2005. ParaView: An End-User Tool for Large Data Visualization, Visualization Handbook, Elsevier, Burlington, Massachusetts, USA.
6 Malkiewicz, A., & Dziedzic, M., 2012. Bone marrow reconversion – imaging of physiological changes in bone marrow. Polish Journal of Radiology. 77(4):45-50.
7 Craig, A.S., Birtles, M.J., Conway, J.F., Parry, D.A. 1989. An estimate of the mean length of collagen fibrils in rat tail-tendon as a function of age. Connect. Tissue Res. 19, 51-62.
Figures