1422
MRI Cytography: a biomarker of microstructural myofiber damage in Amyotrophic Lateral SclerosisNatenael B Semmineh1, Alberto Fuentes1, David Medina1, Rachael Sirianni1, and C Chad Quarles1
1Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
For patients diagnosed with Amyotrophic Lateral Sclerosis (ALS), the clinical heterogeneity of disease presentation and progression continues to confound the identification of robust outcome measures and biomarkers that can be used as surrogates of progression to provide faster and improved decision-making during clinical trials. To overcome this limitation we developed a non-invasive imaging strategy, termed MRI Cytography (MRC) that is uniquely sensitive to abnormal muscle cytoarchitecture. In a preclinical model of ALS, MRC was able to reliably differentiate between normal and degenerated muscle microstructure.
Introduction
Amyotrophic Lateral Sclerosis (ALS) is characterized by loss of spinal and cortical motor neurons, resulting in progressive muscle atrophy. The clinical heterogeneity of disease presentation and progression continues to confound the identification of robust outcome measures and biomarkers that can be used as surrogates of progression to provide faster and improved decision-making during clinical trials. To overcome this limitation we developed a non-invasive imaging strategy, termed MRI Cytography (MRC), that is uniquely sensitive to abnormal muscle cytoarchitecture (e.g. atrophy, reduced fiber diameter, fiber atypia, fiber density). In this study, we describe the first evaluation of MRC in the context of a mouse model of ALS.Methods
MRC is based on susceptibility based contrast enhanced MRI and is similar to a multi-echo gradient echo (GRE) DSC-MRI perfusion approach. However, with MRC the focus is not on characterizing tracer kinetics but rather quantifying and mapping the transverse relaxivity at tracer equilibrium (TRATE) [1]. A multi-echo GRE pulse sequence is used to quantify the T2* and T1 changes (ΔR2*(t) and ΔR1(t)) that occur after the injection of a contrast agent (CA). The voxel-wise CA T2* relaxivity can then be computed at any time point using ΔR2* / Ct. Similar to DCE-MRI, the CA concentration (Ct) is computed using ΔR1/r1, where r1 is the CA’s (known) T1 relaxivity. When computed at CA equilibrium the relaxivity is what is termed TRATE. Exogeneous CAs compartmentalize around myofibers in muscle and any induced magnetic field perturbations (due to susceptibility differences between the intra- and extrafiber space) will predominantly reflect the myofiber cytoarchitecture (density, size, shape) and will be reflected in the derived TRATE values. Our hypothesis is that ALS-induced fiber degeneration, which is known histologically to reduce myofiber diameters, will lead to decreases in TRATE values. To test this hypothesis, we compared TRATE values in the hind limb muscles of wild type mice (n=4) and the SODG93A mouse model of ALS (n=4). Histopathology was used to characterize the microstructure.Results
As shown in Figure 1, the sensitivity of contrast enhanced GRE (ΔR2*) and spin echo (ΔR2) data to perturber sizes over the range of myofiber diameters (20 – 100 μm) demonstrate the biophysical basis of MRC. We have also previously shown that, at CA equilibrium, the measured ΔR2* values, and by extension TRATE, exhibit a strong dependency on the underlying tissue microstructure, such as cell size and density [1]. we characterized GRE based TRATE values across the hind limb muscles of wild type and SODG93A mice. Consistent with prior studies, the mean muscle fiber diameter in the SODG93A mice decreases more than 30% as compared to wild type controls. The voxel-wise TRATE values decreased similarly, with a particularly marked reduction (> 50%) in the top quartile of the histogram. It is also worth noting that the measured TRATE values were up to nearly 200 mmol-1s-1, indicating that the microstructural nature of muscle is conducive to the development of strong, contrast agent induced susceptibility effects. The magnitude of these mesoscopic CA effects far exceed the T1 and T2 microscopic relaxivity of the CA (~4 mmol-1s-1).Discussion
Our prior computational work [1] revealed that TRATE decreases with cell size and density and the reduction in TRATE values observed in the muscles of SODG93A mice is consistent with this finding. While we highlight the GRE data in this feasibility study we are continuing the analysis of the SE data acquired in the same animals. Consistent with Figure 1, we expect that SE-based TRATE values will increase with decreased myofiber size. If the sarcolemma is disrupted, as can occure with severe fiber damage, the GRE and SE based TRATE values could also substantially decrease due to the loss of susceptibility difference between the intra- and extra-fiber space.Conclusion
These studies demonstrate that MRC may have the potential to serve as a quantitative histology-specific outcome measure for ALS, thereby providing a novel approach for assessing disease progression in patients and treatment response in clinical trials.Acknowledgements
Flynn Foundation #2094References
- Semmineh, N. B., Xu, J., Skinner, J. T., Xie, J., Li, H., Ayers, G., & Quarles, C. C. (2015). Assessing tumor cytoarchitecture using multiecho DSC-MRI derived measures of the transverse relaxivity at tracer equilibrium (TRATE). Magnetic Resonance in Medicine, 74(3), 772–784.
Figures
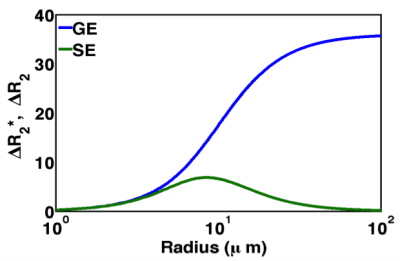
Figure 1: Dependence of gradient echo (GRE)
and spin echo (SE) relaxation rates on perturber size for a given echo time and
perturber geometry (e.g. myofibers).
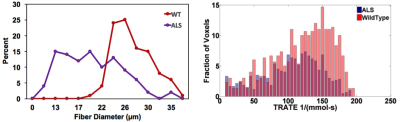
Figure 2: Regional
distribution of myofiber diameters (left) and voxel-wise TRATE values (right) in wild type (n = 4) and SODG93A (n = 4) mice.
Both fiber size and TRATE distributions showed a shift towards smaller
values matching the pathology of ALS and the biophysical basis of TRATE.