1417
Elevated conversion of hyperpolarized [1-13C]pyruvate to [1-13C]lactate is not associated with tissue acidosis, as measured with hyperpolarized [13C]bicarbonate, in a murine model of rheumatoid arthritis.1CRUK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 2Department of Pharmacology, University of Cambridge, Cambridge, United Kingdom, 3Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Synopsis
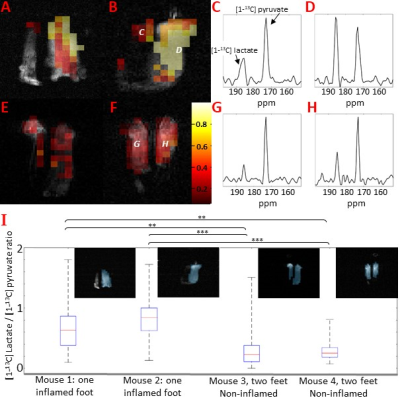
Measurements of synovial fluid pH in patients with rheumatoid arthritis suggest acidosis can occur at inflamed joints. A widely used model of rheumatoid arthritis is produced by injecting complete Freund’s adjuvant into the hind paw of a mouse. We have investigated whether inflammation is associated with acidosis in this model using Magnetic Resonance Spectroscopic Imaging of injected hyperpolarised [1-13C]pyruvate, to detect the metabolic changes associated with inflammation, and hyperpolarised [13C]bicarbonate to measure extracellular pH. A significant increase in the [1-13C]lactate/[1-13C]pyruvate was observed throughout the inflamed tissue, but there is no apparent acidosis
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease in which joints become inflamed and is associated with leukocyte invasion, synoviocyte hyperplasia, pannus formation, and most importantly from a patient’s viewpoint, pain. Patients with RA can show increased lactate concentrations and acidosis of the synovial fluid, though the measured synovial pH varies between patients [1]. We used a model of RA in single hind paws of mice to investigate whether the increased-lactate production associated with inflammation was accompanied by acidosis. 13C Magnetic Resonance Spectroscopic Imaging (MRSI), following injection of hyperpolarised [1-13C]pyruvate, showed increased glycolytic activity in inflamed joints, as has been observed previously in a rat CFA-inflammation model [2]. 13C MRSI measurements with hyperpolarised [13C]bicarbonate were then used to measure extracellular pH in these inflamed tissues. These measurements - in an established model of inflammatory pain [3] - allowed us to investigate whether pain could be caused by low pH stimulation of acid-sensing sensitive ion channels expressed by sensory neurons.METHODS
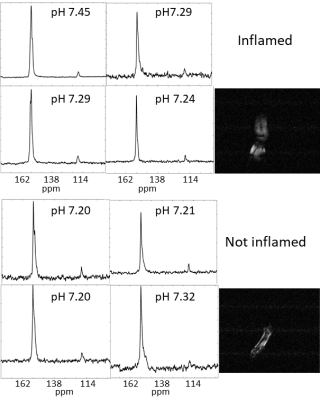
Experiments were conducted in accordance with the United Kingdom Animal (Scientific Procedures) Act 1986. Mice were anaesthetised using isofluorane (2%) and two 15 μl injections of 10 mg/ml Complete Freund’s Adjuvant (CFA, Chondrex) were made, to give a total dose of 300 µg/paw. Paw swelling was measured with calipers for five to seven days [3]. Images and spectra were acquired using a 7T MR instrument (Agilent, Palo Alto, CA, USA). Proton images were acquired axially through downwards pointing feet using a volume coil. Images were acquired using a fast spin echo (FSE) sequence (40 x 40 mm slices, 2 and 6-10mm thick, covering the entire foot, 128 x 128 data points, 8 echoes, effective echo time (TE) of 48ms, 2s repetition time (TR)) or using a spoiled gradient echo (GE) sequence (40 x 40 mm, 2mm thick slices; 128 x 128 data points; TR, 400ms; TE 2.85ms). Hyperpolarised [1-13C] pyruvate solution was polarised using a Hypersense dissolution Dynamic Nuclear Polariser (Oxford, UK), as described in [4]. 13C MRSI data were acquired from both non-inflamed hind paws of 2 mice 20s after injection of hyperpolarized [1-13C]pyruvate, 1 day prior to CFA-injection and from 2 mice 5-7 days post injection. Hyperpolarised [13C]bicarbonate was prepared and injected as described previously [5]. Single shot pulse acquire MR spectra were acquired from mouse hind paws using a 9 mm diameter solenoid; 4 non-inflamed paws and 4 (5-7day) CFA-inflamed paws. Spectra were acquired every second, from 12s after injection to allow equilibration of hyperpolarized 13C label between bicarbonate and carbon dioxide. The first 28 spectra were summed, phase corrected and baseline corrected. The 13CO2/H13CO3 ratio was calculated by integrating the signal intensities between 127 - 123ppm and 163 – 156ppm respectively and converted to a pH value using the Henderson-Hasselbach equation, assuming a pKa of 6.17 [5].RESULTS
Following injection of CFA, inflammation of the ankle and footpad was significantly greater than in control paws from days 1 to 7 (p < 0.0001, n = 5 mice). There was a significant elevation of the [1-13C]lactate/[1-13C]pyruvate ratio in the CFA-inflamed paws (Figure 1) compared to non-inflamed paws. The mean lactate-to-pyruvate ratios were 0.30 ± 0.23 for non-inflamed paws and 0.74 ± 0.32 for CFA-inflamed paws (p < 0.001, for individual comparisons: see Figure 1). The spectra and pHs calculated from the 13CO2/H13CO3 ratios are shown in Figure 2. The pHs in CFA-inflamed and non-inflamed paws were not significantly different (pH 7.32 ± 0.09 versus pH 7.23 ± 0.06 respectively, n = 4, p = 0.92).DISCUSSION
An Increased [1-13C]lactate/[1-13C]pyruvate ratio, which is indicative of increased glycolytic activity, was observed throughout the CFA-inflamed heel and foot. MacKenzie et al. [2] showed marked infiltration of leukocytes in rat feet with CFA-induced inflammation. Leukocytes increase glycolysis on activation as do stromal cells in inflammation, such as fibroblast-like synoviocytes in RA [6]. However, despite widespread inflammation, which was evident throughout the heel and foot, there was no apparent reduction in extracellular pH. This result may explain why mice that lack genes encoding acid-sensing ion channels (ASIC) 1, 2 and 3 do not display diminished pain behaviour in the CFA model, i.e. acidosis is not a significant factor in this model.CONCLUSION
The CFA-inflamed mouse paw model of arthritis shows increased lactate labelling, indicative of increased glycolytic activity, but this is not accompanied by a significant decrease in pH. These data suggest that tissue acidosis may not be a primary contributor to the pain observed [3] in the CFA-induced arthritis model.Acknowledgements
EStJS, ZMAH and GC are grateful to funding from Arthritis Research UK (Grant Reference 20930) and KMB, AJW and DH to Cancer Research UK Programme: Grant number: 17242.References
[1] Fujii W, Kawahito Y, Nagahara H, et al. Monocarboxylate Transporter 4, Associated With the Acidification of Synovial Fluid, Is a Novel Therapeutic Target for Inflammatory Arthritis. Arthritis & Rheumatology. 2015; 67(11): 2888-2896.
[2] MacKenzie JD, Yen Y-F, Mayer D, Tropp JS, Hurd RE, Spielman DM. Detection of Inflammatory Arthritis by Using Hyperpolarized 13C-Pyruvate with MR Imaging and Spectroscopy. Radiol. 2011;259(2):414-420.
[3] Chillingworth NL, Donaldson LF. Characterisation of a Freund’s complete adjuvant-induced model of chronic arthritis in mice. J Neurosci Meth. 2003; 128(1-2):45-52.
[4] Serrao EM, Rodrigues TB, Gallagher FA, Kettunen MI, Kennedy BWC, Vowler SL, Burling KA, Brindle KM. Effects of fasting on serial measurements of hyperpolarized [1‐13C]pyruvate metabolism in tumors NMR Biomed. 2016; 29(8): 1048–1055.
[5] Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008; 453(7197): 940-943.
[6] Garcia-Carbonell R, Divakaruni AS, Lodi A, et al. Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis & Rheumatology. 2016; 68(7): 1614-1626.
Figures