1401
Measurement of skeletal muscle extraceullar volume (ECV) in the healthy thigh: determination of the time to contrast equilibrium1Department of Medical Physics & Engineering, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 2NIHR Leeds Biomedical Research Centre, Leeds, United Kingdom, 3Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 4Department of Biomedical Imaging Science, University of Leeds, Leeds, United Kingdom
Synopsis
Five healthy volunteers were scanned at 3 T to determine the time to contrast equilibrium in skeletal leg muscle to establish whether extracellular volume (ECV) mapping is clinically feasible for skeletal muscle (as it has proved to be for myocardium). Time to contrast equilibrium was 13 minutes, and native T1 values were validated against the literature. It was also found that the difference in measurement of ECV using the aorta compared to the femoral artery was small. It is hoped that advancements in this technique could aid in the diagnosis and treatment of scleroderma patients with muscle involvement.
Introduction
Over recent years, extracellular volume (ECV) measurement of the myocardium by T1 mapping with gadolinium-based contrast agents has aided in the diagnosis of cardiomyopathy and other myocardial pathologies1. There is some evidence suggesting that ECV changes can also be detected in skeletal muscle which can help understand how muscles are affected in patients with systemic inflammatory diseases (e.g. scleroderma2). For accurate ECV measurements, the contrast administration protocol and image timings must be chosen such that post-contrast data is acquired after contrast equilibrium has been established between the blood plasma and the interstitium of the tissue of interest. This study aimed to assess the time to contrast equilibrium in the thigh muscle of healthy volunteers. As blood measurements taken from the femoral artery in the thigh may be susceptible to partial volume and/or in-flow effects, a comparison was made with reference measurements taken from the descending aorta to establish whether femoral artery based blood measurements are adequate.Methods
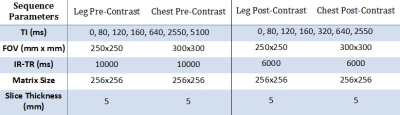
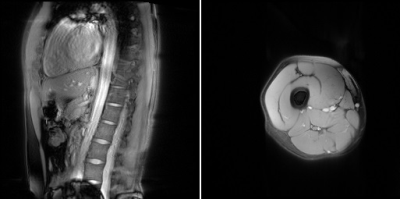
Five healthy volunteers were scanned on a 3 T Verio scanner (Siemens, Erlangen, Germany). Volunteers were imaged with a body matrix coil over their chest, and two small flex-coils surrounding their thigh, located 4 cm above the patella. Pre-contrast inversion recovery 2D TrueFISP scans (Table 1) of both the upper leg (transverse section) and the descending aorta (oblique-sagittal section, Figure 1) were acquired. The volunteers then received a bolus injection of 0.1 mmol/kg (maximum 20 ml) Dotarem (gadoterate meglumine, Guerbet LLC, USA), and the scans were repeated contiguously for approximately 40 minutes post injection alternating between the leg and aorta fields of view, interleaving the scans which gave a temporal resolution of approximately 3 minutes. IR-TR was reduced post-contrast to reflect the reduction in T1 from contrast agent administration.
T1 was estimated using a least-squares approach to the inversion recovery model:
$$S=S0(1-λ.exp[-TI/T1])$$
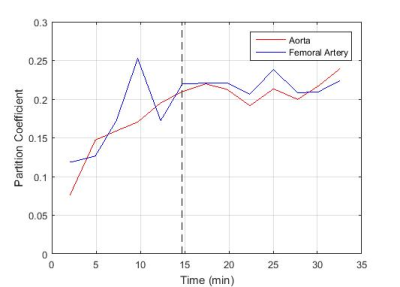
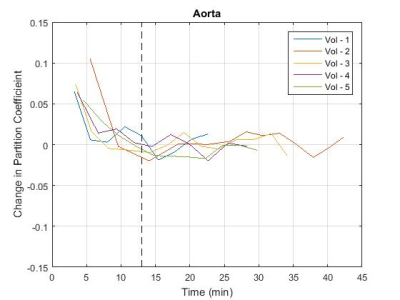
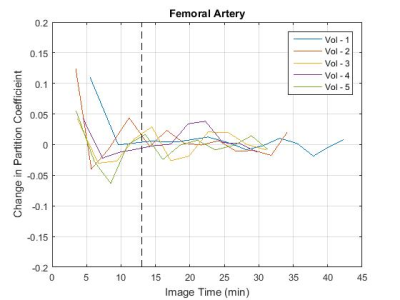
S is the mean signal within an ROI drawn inside the tissue or vessel, S0 is the signal acquired after complete longitudinal recovery, λ is the magnetisation preparation efficiency, and TI is the inversion time. S0, λ, and T1 were all free variables in the fit with S0 and T1 constrained to positive numbers and λ constrained between 0 and 2 (2 representing ideal inversion). The partition coefficient (ΔR1,muscle/ΔR1,blood) was calculated at the time of each post-contrast leg scan, with the aorta ΔR1 values linearly interpolated to the time of the leg scan. The partition coefficient values were plotted (Figure 2) and the time to contrast equilibrium was visually assessed as the time at which the variation in calculated partition coefficient plateaued. The change in the partition coefficient was also plotted (Figure 3) as an aid in determining this value. This was repeated using blood T1 data from the femoral artery to assess the feasibility of performing the analysis using blood measurements from the thigh image. The mean time to contrast equilibrium for all volunteers was then calculated.
Results
The measured native T1 values were 1395 ± 13ms for muscle, 1815 ± 64ms for aortic blood and 1798 ± 96ms for blood in the femoral artery. These values also include data taken from three additional volunteers scanned without contrast administration for sequence development. The mean time to contrast equilibrium was determined to be 13 min (±2 min, 10-21 min) (mean ± SD range). The partition coefficient measured at this time was 0.15 (±0.02, 0.13-0.17) for blood measurements taken from the aorta, and 0.18 (±0.04, 0.15-0.23) for blood measurements taken from the femoral artery. At the equilibrium time point, the mean partition coefficient measured using the aorta was 0.03 (±0.03, 0.00-0.07) lower than that measured using the femoral artery. This would lead to a difference in ECV measurement of approximately -2% (assuming 44% haematocrit3).Discussion
The measured T1 values are comparable to those from the literature (blood T1: 1664 ± 14ms 4, 2076 ± 125ms 5, muscle T1: 1420 ± 38ms 6, 1412 ± 13ms 7). Time to contrast equilibrium of 13 mins is comparable to that used in CMR (10-30 minutes)8, and was consistent across all volunteers, implying that these measurements would be feasible within the time constraints of a standard clinical scan.Conclusion
The mean time to equilibrium was 13 minutes and there was no significant difference (p=0.09) in measured partition coefficient between blood measurement taken from the aorta and the femoral artery. Skeletal muscle ECV measurements are feasible within a clinically realistic time scale and can be performed using within slice blood measurements in the healthy thigh.Acknowledgements
We would like to thank Brian Chaka, our radiologist at Chapel Allerton, for his tireless work performing the MR acquisitions on all of our volunteers, and all the volunteers who agreed to be scanned for our research. It would not have been possible without them.
Dr J D Biglands is funded by a National Institute for Health Research (and Health Education England), Clinical Lectureship. This paper presents independent research funded by the National Institute for Health Research (NIHR) and Health Education England.
References
[1] M. Ugander, A. J. Oki, L.-Y. Hsu, P. Kellman, A. Grieser, A. H. Aletras, C. T. Sibley, M. Y. Chen, W. P. Bandettini and A. E. Arai, “Extracellular Volume Imaging by Magnetic Resonance Imaging Provides Insight into Overt and Sub-Clinical Myocardial Pathology,” European Heart Journal, vol. 33, pp. 1268-78, 2012.
[2] A. Barison, L. Gargani, D. De Marchi, G. D. Aquaro, S. Guiducci, E. Picano, M. M. Cerinic and A. Pingitore, “Early Myocardial and Skeletal Muscle Interstitial Remodelling in Systemic Sclerosis: Insights from Extracellular Volume Quantification using Cardiovascular Magnetic Resonance,” vol. 16, no. 1, 2015.
[3] M. S. Weatherall and K. M. Sherry, “An evaluation of the Spuncrit (TM) Infra-red Analyzer for Measurements of Haematocrit,” Clin Lab Haem, vol. 19, pp. 183-6, 1997.
[4] H. Lu, C. Clingman, X. Golay and P. C. van Zijl, “Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla,” Magn Res Med, vol. 52, no. 3, pp. 679-82, 2004.
[5] C. T. Sibley, J. Huang, M. Ugander, A. Oki, J. Han, M. S. Nacif, A. Greiser, D. R. Messroghli, P. Kellman, A. E. Arai, D. A. Bluemke and S. Liu, “Myocardial and blood T1 quantification in normal volunteers at 3T,” Journal of CMR, vol. 13, no. Suppl 1, p. 51, 2011.
[6] G. E. Gold, E. Han, J. Stainsby, G. Wright, J. Brittain and C. Beaulieu, “Musculoskeletal MRI at 3.0 T: relaxation times and image contrast,” AJR AM J Roentgenol, vol. 183, no. 2, pp. 343-51, 2004.
[7] G. J. Stanisz, E. E. Odrobina, J. Pun, M. Escaravage, S. J. Graham, M. J. Brionskilll and R. M. Henkelman, “T1, T2 relaxation and magnetization transfer in tissue at 3T.,” Magn Res Med, vol. 54, no. 3, pp. 507-12, 2005.
[8] D. R. Messroghli, J. C. Coom, V. M. Ferreira, L. Grosse-Wortmann, T. He, P. Kellman, J. Mascherbauer, R. Nezafat, M. Salerno, E. B. Schelbert, A. J. Taylor, R. Thompson, M. Ugander, R. B. van Heeswijk and M. G. Freidrich, “Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR)...,” Journal of Cardiovascular Magnetic Resonance, vol. 19, no. 75, 2017.
Figures