1396
Multi-centric evaluation of stability of quantitative outcome measures in healthy calf muscles1Neurology, BG UK Bergmannsheil gGmbH, Bochum, Germany, 2Radiology, Universitiy Medical Center Utrecht, Utrecht, Netherlands, 3C.J. Gorter Center for High Field MRI, Leiden University Medical Center, Leiden, Netherlands, 4Image Science Institute, Universitiy Medical Center Utrecht, Utrecht, Netherlands, 5Brain Centre Rudolf Magnus, Neurology, Universitiy Medical Center Utrecht, Utrecht, Netherlands, 6Radiology, Academic Medical Center Amsterdam, Amsterdam, Netherlands
Synopsis
Clinical feasible, comparable muscle MR-techniques are crucial for monitoring disease progression and therapy in patients with neuromuscular diseases. We developed and evaluated a multi-modal quantitative MR protocol at 3T. Diffusion parameters, water T2 relaxation time and fat-fraction were measured and tested for temporal stability, multicenter reproducibility and covariate influence. Diffusion parameters stabilized after 15 minutes and were comparable between centers. Water T2 decreased 1ms within 1 hour. In dorsal muscles fat-fraction increased slightly, due to a decrease in muscle size. Temporal stability of quantitative parameters was shown and showed that T2 decrease needs to be considered when planning protocols.
Introduction
Quantitative MR imaging techniques play an increasingly important
role as surrogate biomarker for the evaluation of neuromuscular diseases (NMD),
their progression and therapy evaluation. Therefore, information about multicenter
reproducibility1 and temporal stability is essential. The purpose of this study was
to evaluate a fast, multimodal MR-Protocol for quantitative muscle imaging. The
derived quantitative parameters were tested for temporal stability, multicenter
reproducibility and influence of age gender and individual calf muscles.Methods
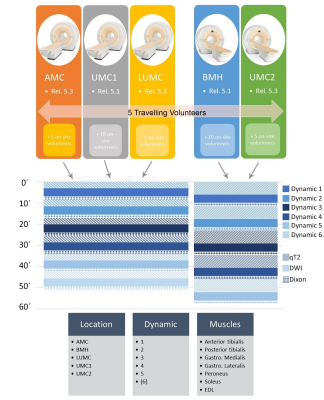
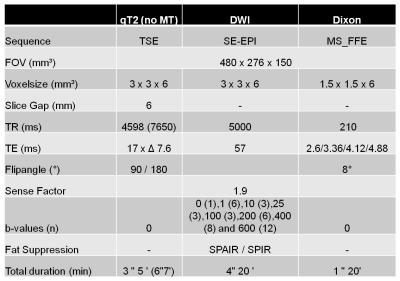
Data from the calf muscles were acquired five to six times within one hour on Philips 3T systems in five different MR-centers (Amsterdam, NL; Bochum, D; Leiden, NL; 2 x Utrecht, NL - Figure 1). In total 40 healthy volunteers (19 males/21 females, age 20-40 years) were included of which five subjects (3 females / 2 males) were examined in all centers (see Figure 1). The protocol included a quantitative 17-echo T2 scan, a diffusion weighted sequence with 42 gradient orientations and a 4-point Dixon sequence (see Figure 2).
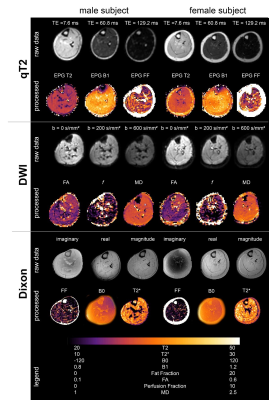
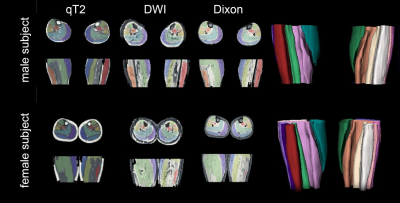
All data processing was done using DTITools (https://github.com/mfroeling/DTITools, Mathematica 11) and was fully automated (Figure 3). The T2 data were fitted using an EPG model2, which handles biases due to B1+ inhomogeneities and calculates separate T2 relaxation times for water (T2w) and fat. Diffusion data were fitted to the tensor model, taking intravoxel incoherent motion (IVIM) into account3, using an iterative weighted linear least-square (REKINDLE-iWLLS) algorithm4. Dixon data were processed using the IDEAL approach5 using eight reference fat peaks6. Manual muscle segmentation of seven muscles per leg was done in 3DSlicer using the Dixon data (Figure 4). Average values of T2w, fat fraction (FF), muscle volume and diffusion parameters for each muscle in the calf were calculated. Temporal stability within one hour scanning time was tested with one-way repeated measured ANOVA and two-way random Intra Class Correlation coefficient (ICC). Multicenter reproducibility of the travelling volunteers was assessed by 2-way mixed ICC. Age, gender and individual muscles were tested for covariance using correlation analysis.
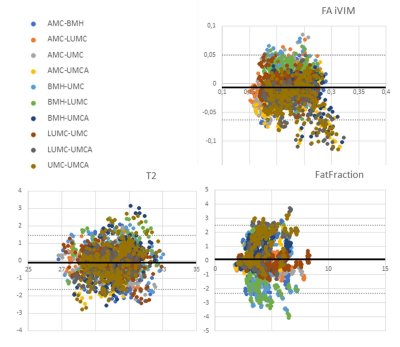
Results
All scans were successfully completed and well tolerated by all volunteers. No data was excluded during processing. Repeated measures ANOVA showed significant (p<0.001) temporal effects for all parameters and sites. Post-hoc tests showed significant differences in: i) FF and muscle volume between the first and last acquisition (ΔFF = 0.04±0.005% and ΔVolume = -1.08±0.06 mm³), ii) FA between first and second acquisition (ΔFA = -0.004±0.0001), and iii) T2w decreased constantly between all acquisitions (ΔT2AMS = ‑0.95ms, ΔT2Boc = -0.03ms, ΔT2Lei = -0.73ms, ΔT2UT1 = -0.79ms, ΔT2UT2 = ‑0.43ms between first and last acquisition). For the five traveling volunteers, multicenter reproducibility showed significant (p<0.0001) agreement for diffusion parameters FA (ICCFA = 0.884), T2w (ICCT2 = 0.909) and FF (ICCfat = 0.835), see Figure 5 for Bland-Altman plots. The 1-way random ICC analysis for multicenter reliability also showed significant good7 agreement between centers for the remaining random volunteers (ICCFA = 0.768, ICCT2 = 0.665 and ICCfat = 0.688). The tested factors age, gender and muscles all show significant correlations for FA, T2w and fat fraction.Discussion
Quantitative imaging parameters of muscle volume, fat fraction, T2w, and diffusion all have a small temporal dependence within a scan time of one hour. T2w decreased up to 1 ms within an hour, which is in the same range as reported treatment effects8. This should be considered, when designing scanning protocols, especially when scans are repeated due to motion artifacts. The changes in muscle volume and FF were very small and mainly noted in the posterior compartment, and could therefore be attributed to muscle compression, resulting in a shift of fluids from the muscle compartment9. Our results also show that data are comparable and repeatable between centers with scanners from the same vendor and within scans. The lower ICC for the FF between centers may be caused by only including healthy volunteers with low FF. Due to low FF, Dixon may be less reliable and slight changes in FF between scan sessions may have a large influence on obtained ICCs. Finally, the significant influence of covariates shows that a control group should be age and gender matched, and that muscles should be observed separately, which is in line with previous findings10.Conclusion
We show that with the applied acquisition protocol, quantitative parameters for muscles can be compared between centers. The timing of the scanning protocol plays a role; therefore, a standardized protocol should be applied, especially when assessing longitudinal patient data. Proper segmentation of the muscles is important, due to large variations between muscles.Acknowledgements
LS was funded by the RUB Research School+ Program.
The authors thank all volunteers for participating.
References
1. Willcocks, R. J. et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann. Neurol. 79, 535–547 (2016).
2. Marty, B. et al. Simultaneous muscle water T 2 and fat fraction mapping using transverse relaxometry with stimulated echo compensation. (2016).
3. De Luca, A., Bertoldo, A. & Froeling, M. Effects of perfusion on DTI and DKI estimates in the skeletal muscle. Magn. Reson. Med. 0, (2016).
4. Veraart, J., Sijbers, J., Sunaert, S., Leemans, A. & Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 81, 335–346 (2013).
5. Reeder, S. B. et al. Iterative Decomposition of Water and Fat With Echo Asymmetry and Least-Squares Estimation ( IDEAL ): Application With Fast Spin-Echo Imaging. Magnenetic Reson. Med. 644, 636–644 (2005).
6. Triplett, W. T. et al. Chemical shift-based MRI to measure fat fractions in dystrophic skeletal muscle. Magn. Reson. Med. 72, 8–19 (2014).
7. Cicchetti, D. V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 6, 284–290 (1994).
8. Arpan, I. et al. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology 83, 974–80 (2014).
9. Lorbergs, A. L., Noseworthy, M. D. & MacIntyre, N. J. Age-related differences in the response of leg muscle cross-sectional area and water diffusivity measures to a period of supine rest. Magn. Reson. Mater. Physics, Biol. Med. 28, 279–290 (2015).
10. Schlaffke, L. et al. Diffusion Tensor Imaging of the Human Calf : Variation of Inter- and Intramuscle-Specific Diffusion Parameters. J Magn Reson Imaging 1–12 (2017).
Figures