1384
Automated segmentation of the cartilage from high-resolution isotropic T1rho MRI1Radiology, New York University School of Medicine, New York, NY, United States, 2Radiology, New York Unversity School of Medicine, New York, NY, United States
Synopsis
We analyze the accuracy of atlas-based cartilage segmentation from isotropic T1ρ MRI and compare it to semi-automated "seed and blanket" method and manual segmentation (ground truth). Reference 3D cartilage masks were taken as the consensus of two human experts. For patella, our implementation of template matching yielded the root mean square volume measurement error RMSE of 0.66 cm3, with interclass correlation coefficient (ICC) = 0.765 and sufficient precision to detect the gender effect. Over two-fold improvement in accuracy, RMSE = 0.25 cm3 and ICC = 0.960 was achieved with a fast, semi-automated algorithm. Similar results hold for the accuracy of the average thickness of segmented masks.
INTRODUCTION
The most promising MRI metrics of Osteoarthritis (OA) of the knee are T2- and T1ρ-relaxation times, both shown to characterize the biochemical composition of articular cartilage1-4. One of the obstacles to their clinical adoption is the need for complex image segmentation. Recent publications demonstrate encouraging results for cartilage segmentation techniques, including active shapes5-6, textures7 and atlas matching8-9. However, there is limited data on segmentation accuracy10. We analyze the accuracy of atlas-based cartilage segmentation from low S/N isotropic T1ρ-weighted images and compare it to semi-automated "seed and blanket" method11.METHODS
T1ρ images of 10 healthy young volunteers (50% female) were acquired on a 3T Siemens Prisma using: TR/TE=1500/4 ms, FA = 8˚, GRAPPA 3, 224×224×192 matrix, 160 mm2 FOV, isotropic 0.71 mm voxels, ten TSL ranging 2-55 ms, TA=22:53 min.
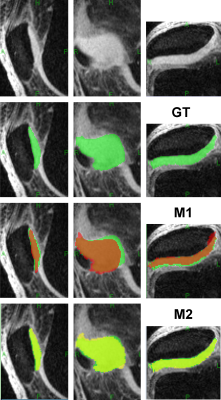
Reference 3D cartilage masks were the consensus of two human experts. This pilot was restricted to the patellar subregion of the cartilage.
Automated atlas matching (M1): The reference atlas consisted of TSL=2 ms volume of one subject and corresponding manual masks. The atlas was then deformed to images of other subjects using a novel mutual-information coregistration system: 1) The transform T is estimated from a neighborhood of the cartilage (morphologic 6-voxel dilation of atlas mask), thus ignoring confounding signal from remote muscle, fat and bone. 2) Iterative optimization of image similarity measure is supplemented by multi-dimensional gridding of initial values for T.
Semi-automated seed and blanket11 (M2): Observer traces over-inclusive cartilage contours on every 5th slice. Contours are interpolated to form the "blanket" (Figure 1a). The desired properties of the blanket: 1) the entire cartilage subregion is included; 2) the adjacent cartilage is excluded. We then apply non-uniformity correction (N3 algorithm, 75 iterations, 10 mm FWHM bias field smoothness). The seed sample of pure cartilage ─ a 10 x 10 voxel tile that optimizes high signal and low variability, is then automatically constructed. In the final step the average seed signal is used for intensity thresholding within the blanket region.
Average thickness algorithm: Regional thickness measures are important for functional analysis of cartilage and bony structures. The thickness of segmented mask is first defined at each internal point Pi as the minimum distance to the nearest surface point12, then averaged over all Pi in the mask.
The fully parallel segmentation software was implemented in Visual C++.
RESULTS
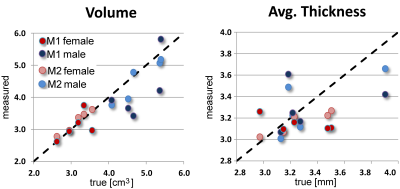
Ground truth: Cartilage volume (patella) ranged 2.62-5.39 cm3; average thickness ranged 2.972-3.904 mm. There was a highly significant effect of gender on volume (t-test, p<0.001, mean male - female difference = 1.67 cm3) but cartilage thickness showed no statistically significant gender effect.
Automatic M1 method: The root mean square volume measurement error RMSE was 0.66 cm3 or 16.6%. There was a good agreement between M1 estimate and the true volume, with interclass correlation coefficient (ICC) = 0.765, 95% confidence interval (C.I.) [0.326-0.935]. For thickness, RMSE was 0.286 mm or 8.6% but ICC was poor: 0.156, with 95% C.I. [0.000-0.695]. A significant gender effect on volume and lack of thickness effects held for M1 estimates. It took less than 1 min CPU time (Intel I7-3930K, 3.2 GHz, 6 cores) to automatically segment each cartilage region using 3D template matching.
Semi-automatic M2 method: Volumetric RMSE was 0.25 cm3 or 6.4%. There was an excellent agreement between M2 estimate and the true volume, with ICC = 0.960, 95% C.I. [0.855-0.990]. Based on paired t-test, mean absolute errors from M2 method were significantly lower than from automated M1 method (t Stat = 1.84, df=9, p=0.04). For thickness, ICC was 0.721, with 95% C.I. [0.251-0.921] and RMSE = 0.179 mm or 5.4%. The gender effect on volume and lack of thickness effects also held for M2 estimates. Semi-automated segmentation took 1-2 min of user intervention, mostly to outline the blanket region.
DISCUSSION & CONCLUSIONS
Segmentation of high-resolution T1ρ MRI can be derived directly from T1ρ images and used for biochemical evaluation of knee articular cartilage. Our implementation of template matching appears reasonably accurate, with precision sufficient to detect the gender effect. Significant improvement in accuracy, however, is possible by using a semi-automated seed and blanket algorithm, at a cost of user interaction (of the order of minutes per case). A larger study, including OA subjects with cartilage defficiencies and analysis of follow-up exams will be needed to better interpret segmentation errors and establish the clinical utility of isotropic T1ρ MRI.Acknowledgements
This work was supported by NIH grants R01-AR060238, R01-AR067156 and R01 AR068966) and performed under the rubric of the Center for
Advanced Imaging Innovation and Research (www.cai2r.net), a NIBIB Biomedical
Technology Resource Center (NIH P41 EB017183).
References
1. Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1ρ and T2 MRI. Osteoarthr Cartil. 2011;19: 171–9.
2. Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: Correlation with biochemical measurements and histology. Magn Reson Imag 2011;29: 324–334.
3. Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—a 3.0-Tesla MRI study. Eur Radiol 2009;19: 132–43.
4. Wang L, Chang G, Xu J, Vieira RLR, Krasnokutsky S, Abramson S, et al. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81: 2329–36.
5. Fripp J, Crozier S, Warfield SK, Ourselin S. Automatic segmentation and quantitative analysis of the articular cartilages from MRI of the knee. IEEE Trans Med Imaging 2010; 29(1):55–64.
6. Vincent G, Wolstenholme C, Scott I, Bowes M. Fully automatic segmentation of the knee joint using active appearance models. Med Image Anal Clin: A Grand Challenge. 2010; 24–230.
7. Dodin P, Pelletier JP, Martel-Pelletier J, Abram F. Automatic human knee cartilage segmentation from 3-D magnetic resonance images. IEEE Trans Biomed Eng. 2010; 57:2699–2711.
8. Shan L, Zach C, Charles C, Niethammer M. Automatic atlas-based three-label cartilage segmentation from MR knee images. Medical Image Analysis. 2014; 18(7):1233–124.
9. Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel based relaxometry. JMRI 2016; 43(4): 970–980.
10. Carballido-Gamio J, Majumdar S. Atlas-based knee cartilage assessment. Magn Reson Med 2011; 66(2):574–83.
11. Rusinek H, Lim J, Wake N, Seah J, Botterill E, Farquharson S, Mikheev A, Lim R. A semi-automated "blanket" method for renal segmentation from non-contrast T1-weighted MR images. MAGMA 2016; 29(2):197-206.
12. Subburaj K, Patil S, Ravi B. Voxel-based thickness analysis of intricate objects. International Journal of CAD/CAM 2006; 6(1):105–115.
Figures