1378
CS+SENSE for Fast UTE Knee Imaging: Technical Feasibility1Radiology, New York University, New York, NY, United States, 2Siemens Healthineers USA, New York, NY, United States
Synopsis
Ultrashort echo time (UTE<1ms) imaging has advantages over traditional long TE (>10ms) imaging to detect asymptomatic (subclinical) cartilage damages in the knee joint, such as fissuring, fracturing and collagen fiber breakdown. To advance UTE imaging toward clinical use, its long scan time needs to be reduced to meet clinical requirement of short protocols. Compressed sensing (CS) and sensitivity encoding (SENSE) parallel imaging have the potential to do so. However, individual use of them has limitations. A combined use of both techniques has been shown in dynamic imaging to be able to achieve higher acceleration factor without SNR loss. This study explores the technical feasibility to extend CE+SENSE to static UTE imaging.
INTRODUCTION
Ultrashort echo time (UTE<1ms) imaging has advantages over traditional long TE (>10ms) imaging to detect asymptomatic (subclinical) cartilage damages in the knee joint, such as fissuring, fracturing and collagen fiber breakdown1. UTE imaging acquires fast-T2-relaxing MR signals and has the capability to visualize cartilage itself without the need of fluid accumulation as a biomarker used in clinical MRI. Noninvasive assessment of subclinical cartilage damages is critically important to biomarker development for diagnosis of early stage osteoarthritis (pre-OA)2,3. To advance UTE imaging toward clinical use, we need to reduce scan time from current ~10min to <5min to meet clinical requirement of short protocols. Compressed sensing (CS) and sensitivity encoding (SENSE) parallel imaging have the potential to do so. However, individual use of them has limitations. CS has an acceleration factor limited to ~4x number of the k-space samples4,5, while SENSE increases noise and reduces SNR6,7. A combined use of both techniques has been proven in dynamic imaging to be able to achieve higher acceleration factor without SNR loss5,8. This study explores the technical feasibility to extend CE+SENSE to static UTE imaging.METHODS
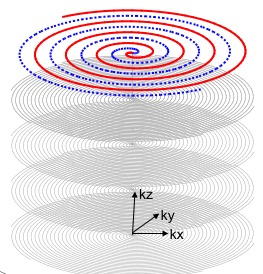
UTE imaging is performed using our custom-developed fast three dimensional (3D) UTE sequence, acquisition-weighted stack of spirals (AWSOS)9. It samples the k-space in a cylindrical volume (Fig. 1), with a uniform phase encoding in the kz (slice) direction and multiple spiral encodings in the kx-ky plane (in-plane). The parallel imaging SENSE is applied to both kz and kx-ky. The CS is applied to the 3D image with Daubechies 4 wavelet for sparse transform4,5. To test feasibility of the proposed approach, we acquire a fully-sampled k-space data set to reconstruct an image as reference, and then take a sub-set out of the full data set for the CS+SENSE to reconstruct an accelerated image. This process is summarized into an optimization problem Eq. 1, with F is Fourier transform operator, C is coil sensitivity maps estimated via a self-calibration procedure10 and smoothed with a 3D 2nd-order polynomial fitting, s is a sub-set of the k-space raw data depending on SENSE acceleration factors (rkz, rkxy), W is the 3D spatial discrete wavelet transform as a sparse transform, and a is a regulation parameter.
Eq. [1] m = min{ || F C m -s ||2 + a|| W m ||1}
EXPERIMENTS
UTE MRI scans were performed on a clinical scanner at 3T (MAGNETOM Trio Tim, Siemens Medical Solutions, Erlangen, Germany) using an 8 channel knee coil (Invivo Inc., Gainesville, Florida, USA). Fully-sampled k-space data were acquired on seven knee patients with ruptured anterior cruciate ligament (ACL) (age 34±13yr in 22-52yr; 3/4 M/F), with an approved IRB. The AWSOS sequence9 was used for the data acquisitions at FOV=140x140mm^2, matrix size=256x256, slices=60, slice thickness=2mm, TR=60ms, TE=0.6ms, q=30°, spirals per slice encoding = 24, and TA=1min26sec. Undersampled data sets were extracted from the fully-sampled data sets by discarding every other rkz slice encodings and/or rkxy spirals. The images were reconstructed offline with custom-developed programs in C++ for SENSE and in MATLAB (R2015b, The MathWorks) for CS.RESULTS AND DISCUSSION
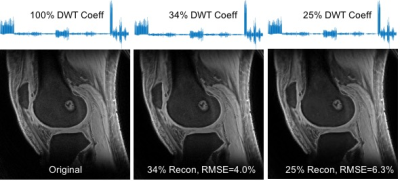
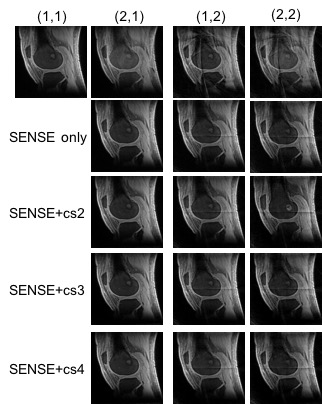
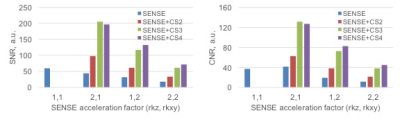
Fig. 2 shows the sparsity of 3D UTE knee images which have a compression rate of up to 4. Fig. 3 presents the images reconstructed at different SENSE and CS acceleration factors and Fig. 4 summarizes the signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) of these images. SNR was measured in the muscle region and signal-free background noise, while CNR was measured in the muscle and femoral bone regions. SNR and CNR were decreasing with SENSE-only acceleration, but increasing with SENSE+CS acceleration. They were restored to the level before any accelerations at CS 3 and 4. These results collectively demonstrated the feasibility of our proposed approach for the acceleration of UTE knee imaging at a factor of 2, 3 or 4, without loss of SNR and CNR of the images.Acknowledgements
This work was financially supported in part by NIH grants R01 CA111996, R01NS082436 and AR052784.References
1. Qian Y, Williams AA, Chu CR, Boada FE. High-resolution ultrashort echo time (UTE) imaging on human knee with AWSOS sequence at 3.0 T. J Magn Reson Imaging. 2012 Jan;35(1):204-10.
2. Lohmander LS. Articular cartilage and osteoarthritis. The role of molecular markers to monitor breakdown, repair and disease. J Anat 1994; 184: 477-492.
3. Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, Bruno S. Quantitative Magnetic Resonance Imaging UTE-T2* Mapping of Cartilage and Meniscus Healing After Anatomic Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2014; 42(8):1847-1856.
4. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007; 58:1182-1195.
5. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med 2010; 64:767-776.
6. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999 Nov;42(5):952-62.
7. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001; 46:638-651.
8. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014; 72:707-717.
9. Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution 3D UTE MR imaging. Magn Reson Med 2008; 60:135-145.
10. Qian Y, Zhang Z, Stenger VA, Wang Y. Self-calibrated spiral SENSE. Magn Reson Med 2004; 52:688-692.
Figures