1368
Differences between neurochemical profiles of male and female C57BL/6 mice1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
The purpose of this study was to demonstrate whether neurochemical profiles of male and female C57BL/6 mice were affected in a sex-related manner. In vivo 1H MRS data were acquired from four different groups of mice, each group consisting of 10 male and 10 female mice. Highly significant differences between male and female groups were consistently observed in each group. These results have serious implications for appropriate quantification referencing (water vs. creatine, male or females in treated vs. control group) for avoiding bias in data interpretation.
PURPOSE
Mouse models of human neurodegenerative diseases are fundamental for preclinical studies. These models require advanced diagnostic methods to characterize adverse pathological processes, to identify efficient biomarkers for monitoring disease progression and for testing new therapeutic strategies. In vivo 1H MRS has been widely used for non-invasive monitoring of brain neurochemistry in longitudinal studies using mouse models of human diseases 1,2. However, there are only a few papers that report sex-related differences in metabolite concentrations quantified from specific brain regions 3. These results raise a number of questions. Can differences in metabolite levels between males and females cause a bias? Which metabolite levels are different between males and females? Are these differences really significant? In order to address these questions, we investigated and evaluated sex-related differences in hippocampal neurochemical profiles of C57BL/6 mice.METHODS
The 1H-MRS data used for this analysis were acquired as part of a different project that required screening of C57BL/6 mice for high brain glutamine 4. Four groups (each group male N = 10, female N = 10) were scanned at either 2 (group #1 and #2) or 3 (group #3 and #4) months of age. Spontaneously breathing animals were anesthetized with 1.0 – 1.5% isoflurane and the body temperature was maintained at 37oC. In vivo 1H MRS data were acquired at 9.4T using FASTMAP shimming 5 and ultra-short TE STEAM (TE = 2 ms) localization sequence combined with VAPOR water suppression 6. Metabolites were quantified using LCModel with the spectrum of fast relaxing macromolecules (MM) included in the basis set and using unsuppressed water signal as an internal reference (assuming 80% brain water content). The two-tailed t-test, combined with the False Discovery Rate (FDR, q = 0.05) method, was used for statistical analysis.RESULTS
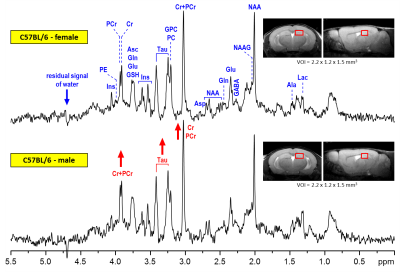
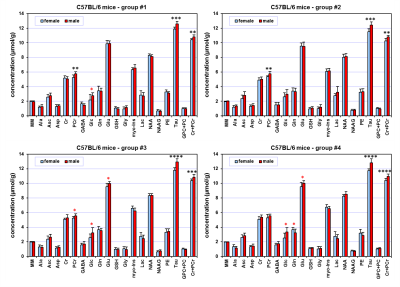
The spectral quality routinely accomplished in this study (Fig. 1) enabled reliable quantification of eighteen brain metabolites and the content of MM. Hippocampal neurochemical profiles quantified from four different mouse groups showed an extremely consistent pattern (Fig 2). Small but highly significant concentration differences were observed between male and female mice. Increased concentrations of taurine (Tau, +0.96 µmol/g on average, p < 0.0002) and total creatine (Cr+PCr, +0.50 µmol/g on average, p < 0.005) were measured in the hippocampus of male relative to female C57BL/6 mice. In addition, increased phosphocreatine (PCr, p < 0.002) was detected in males relative to females at two months of age (group #1 and #2). Additionally, other metabolites such as glucose (Glc) indicated significant differences between male and female groups (t-test, p < 0.05), but did not pass the FDR test for multiple comparisons. When all measured date were pooled together (male N = 40 and female N = 40), significant differences (including FDR correction) between male and female mice were observed for ascorbate (Asc), Glc, PCr, Tau and Cr+PCr.DISCUSSION
The 1H-MRS data of this study demonstrate with very high confidence that hippocampal neurochemical profiles of male and female C57BL/6 mice are different. In general, observed differences are in good agreement with the results reported by Duarte 3, but presented data provide much clearer evidence for sex-related differences in total creatine concentrations in young adult mice. Although the largest differences between male and female mice were observed for Tau (up to 8%), the difference in Cr+PCr (up to 5%) may have more serious implications, because total creatine is still frequently used as an internal reference for metabolite quantification. Obviously, using total creatine as a reference in studies aimed at sex-related differences would cause major bias in quantification of other brain metabolites. Pooling together male and female data is a common practice in 1H-MRS studies, but this approach increases the coefficient of variation of metabolites due to sex-related differences and consequently decreases the chance to detect small differences between treated and control mice. In addition, these data clearly demonstrate that study design protocols using mouse models that do not adequately control for male and females in experimental and control group can lead to major bias in data interpretation.
This study clearly demonstrates that neurochemical changes as small as 0.5 µmol/g can be routinely detectable in the mouse brain by 1H MRS at 9.4T with high confidence. These data also illustrate the importance of FDR correction to reduce the probability of false positive results.
CONCLUSIONS
Sex-related differences in metabolite concentrations, especially those of taurine and total creatine, have to be taken into account for precise neurochemical profiling in the hippocampus of C57BL/6 mice.Acknowledgements
Supported by: NIH grants P41 EB015894, P30 NS076408 and WM KECK FoundationReferences
1. Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed 2007;20(3):216-237. 2. Duarte JM, Lei H, Mlynarik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage 2012;61(2):342-362. 3. Duarte JM, Do KQ, Gruetter R. Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging 2014;35(7):1660-1668. 4. Cudalbu C, McLin VA, Lei H, Duarte JM, Rougemont AL, Oldani G, Terraz S, Toso C, Gruetter R. The C57BL/6J mouse exhibits sporadic congenital portosystemic shunts. PLoS One 2013;8(7):e69782. 5. Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 2000;43(2):319-323. 6. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999;41(4):649-656.Figures