1367
Does maternal swimming during gestation protects the neonatal brain from hypoxic-ischemic injury?1Service développement et croissance, Université de Genève, Geneva, Switzerland, 2Laboratoire d'imagerie fonctionnelle et métabolique, Ecole polytechnique fédérale de Lausanne, Lausanne, Switzerland, 3Institut translationnel d'imagerie moléculaire, Université de Genève, Geneva, Switzerland
Synopsis
There are growing evidences that swimming during gestation has a neuroprotective effect on offspring perinatal brain injuries. The aim of this work was to assess this neuroprotective effect on P3 hypoxic-ischemic model by 1H-MRS and diffusion MRI (DTI and NODDI) at 9.4T. A moderate, but real effect of swimming during gestation on the neurochemical profile 24h after HI was observed. Difference in neurochemical profile between sedentary and swimming rats may lead to a different response to the injury. At long-term, diffusion MRI derived parameters changes following HI were restored in the swimming HI group, providing evidence of a neuroprotective effect.
Introduction
Animal models of preterm
brain injury can be achieved by Hypoxia-Ischemia (HI)1.
There are growing evidences that swimming during pregnancy can have a neuroprotective
effect on offspring perinatal brain injuries, acting on brain metabolism, changing
antioxidant activity as well as influencing brain-derived
neurotrophic factor2. The aim of this work was to assess the
neuroprotective effect of swimming during pregnancy in subsequent HI brain
injury in the newborn rats. 1H-MRS and diffusion MR imaging (Diffusion
Tensor Imaging (DTI) and Neurite Orientation Dispersion and Density Imaging
(NODDI)) were performed at 9.4T.Methods
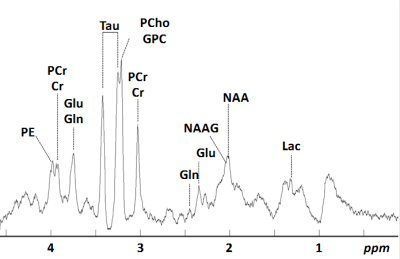
Pregnant Wistar rats were divided in 4 groups: (1) Sedentary-Sham (SE_SH), (2) Sedentary-Hypoxic-Ischemic (SE_HI), (3) Swimming-Sham (SW_SH) and (4) Swimming-Hypoxic-Ischemic (SW_HI). The swimming group was subjected to a swimming protocol: 20 min/day during all gestation whereas the sedentary group did not perform any swimming. At Post Natal Day (P)3 pups from -HI groups underwent moderate HI injury: right carotid artery occlusion followed by 30min at 6% O2. The Sham group was not exposed to occlusion and hypoxia. All MR experiments were performed on an actively-shielded 9.4T/31cm magnet (Varian/Magnex) equipped with 12-cm gradient coils (400mT/m, 120μs). A quadrature transceive 20-mm surface RF coil was used for 1H-MRS. 24h following HI, after automatic FASTMAP shimming, spectra acquisition on a VOI of 1.5×1.5×2.5mm3 within the cortical lesion was performed using an ultra-short echo time (TE/TR = 2.7/4000ms) SPECIAL spectroscopy method3. At P4, 16 series of FIDs (16 averages each) were acquired, individually corrected for frequency drift, summed together and corrected for residual eddy current effects using the reference water signal. Proton spectra were analyzed with LCModel4. At P60, rats were sacrificed and brains were paraformaldehyde-fixed for subsequent ex-vivo MRI with a 2.5 mm diameter birdcage coil. A multi-b-value shell protocol was acquired using a spin-echo sequence (FOV = 21×16mm2, matrix size = 128×92, 12 slices of 0.6mm, 3 averages with TE/TR = 45/2000ms). 96 DWI were acquired, 15 b0 images and 81 separated in 3 shells (non-collinear and uniformly distributed in each shell) with (# of directions/b-value in s/mm2): 21/1750, 30/3400 and 30/5100. Acquired data were fitted using the NODDI toolbox5. Four different brain regions were identified: cortex (Cx), corpus callosum (CC), external capsule (EC) and Striatum (St). DTI derived parameters (Axial diffusivity (AD), Radial diffusivity (RD), Mean diffusivity (AD) and Fractional anisotropy (AD)) as well as NODDI derived parameters (intra-neurite volume faction (fin), isotropic volume fraction (fiso) and orientation dispersion index (ODI)) were averaged in the different regions assessed. For statistics, a Mann Whitney test was used (significance: P<0.05).Results
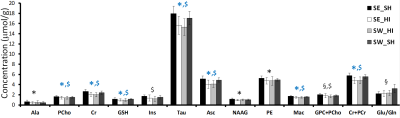
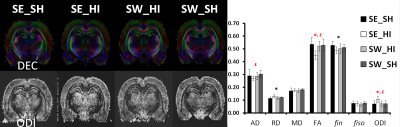
Following HI (P4) most of the metabolites (Fig. 1) were significantly decreased in the SE_HI and SW_HI groups compared to SE_SH rats including PCho, GSH, Tau, Cr. Total Cr, Asc and Mac. NAAG, Ala and PE were significantly decreased in the SE_HI group compared to SE_SH but not in the SW_HI group. In addition, swimming induces changes in the neurochemical profile (Fig. 2) of control animals: a decrease in the total Cho and an increase in the Glu/Gln. At P60 (Fig. 3), in Cx and St (grey matter), only few changes were observed between the groups in the diffusion MRI. In the CC and EC (white matter), significant FA and fin decrease as well as RD and ODI increase were observed in the SE_HI group compared to SE_SH. Interestingly, AD, FA and ODI were also significantly lower in the SE_HI compared to SW_HI group. Not any difference was observed between SE_SH and SW_HI groups providing evidence of the swimming benefit.Discussion and conclusion
In this study we characterized the effect of swimming on acute metabolic (P4-24h after injury) and long-term microstructural (P60) changes following HI at P3. A moderate, but real effect of swimming during gestation on the neurochemical profile 24h after HI was observed. Swimming modifies the neurochemical profile in the sham rats (without HI) acting on Glu/Gln (relative to the Glu-Gln cycle) and Choline (component of cell membrane). Swimming also preserves Ala (nonessential amino acid), NAAG (neuronal marker) and PE (component of cell membrane) evidencing a partial neuroprotective effect following HI. Difference in neurochemical profile between sham rats sedentary versus swimming may lead to a different response in the case of a lesion with different subsequent brain development. At long-term (P60) diffusion MRI derived parameters changes following HI depicted mainly white matter/myelination injury (AD and FA decrease as well as RD and ODI increase) that were restored in the swimming HI group, indicating a neuroprotective effect of gestational swimming on the neonatal pup white matter microstructure.
This study can be of high interest for the neonatologist community providing evidence that the simple fact of swimming during gestation has a potential neuroprotective effect on babies.
Acknowledgements
Supported: by bourses d’excellence de la Confédération Suisse pour chercheurs étrangers, le fond national Suisse n° 33CM30-124101/140334, The Fondation pour Recherches Médicales, the CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, Leenards and Jeantet foundation.References
[1] van de Looij Y et al. MRI of animal models of developmental disorders and translation to human imaging. Cur. Opin. in Neur. 2014 Apr;27(2):157-67. [2] Marcelino TB et al. Evidences that maternal swimming exercise improves antioxidant defenses and induces mitochondrial biogenesis in the brain of young Wistar rats. Neuroscience. 2013 Aug 29;246:28-39. [3] Mlynarìk V et al. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn. Reson. Med. 2006 Nov;56(5):965-70. [4] Provencher SW et al. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993 Dec;30(6):672-9. [5] Zhang H et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012 Jul 16;61(4):1000-16.Figures