1364
Longitudinal follow-up of brain metabolism in rat models of progressive Parkinson's disease using Magnetic Resonance Spectroscopy Imaging.1MRI department, CHU Clermont-Ferrand, Clermont-Ferrand, France, 2IBDM, UMR 7288 CNRS / Aix-Marseille Université, Marseille, France, 3AgroResonance-UR370 QuaPA, Saint Genes Champanelle, France, 4Centre de Résonance Magnétique Biologique et Médicale UMR 7339 CNRS / Aix-Marseille Université, Marseille, France, 5Neurology department, CHU Clermont-Ferrand, Clermont-Ferrand, France, 66Université Clermont Auvergne (UCA), EA7280 NPSY-Sydo, Clermont-Ferrand, France
Synopsis
The development of animal models that reproduce the selective and progressive loss of nigral dopamine neurons characterizing Parkinson’s disease has opened new possibilities to study the disease evolution. Here magnetic resonance spectroscopy imaging was used to follow up the distributions of metabolites in key basal ganglia components in two rat models of progressive parkinsonism at three time points over a period of 120 days following injury. First results on overtime changes in NAA and glutamate repartition will be presented. Completion of this project may provide novel insights onto the pathological alterations associated with the progression of the neurodegenerative process.
Purpose/introduction: The massive and progressive degeneration of the dopamine (DA) neurons in the substantia nigra (SN) pars compacta innervating the basal ganglia is a main pathological hallmark of Parkinson’s disease (PD). Considering the studies that suggest the involvement of glutamate-mediated mechanisms in PD pathophysiology and pathogenesis1,2, a new rat model has been developed based on pharmacologically-induced dysfunction of excitatory amino acid transporters (EAATs). Single unilateral intranigral injection in the rat of the substrate inhibitor of EAATs, L-trans-pyrrolidine-2,4-dicarboxylate (PDC), triggers a neurodegenerative process mimicking several PD features3: i) selective loss of nigra DA versus non-DA neurons, ii) evolution pattern of neurodegeneration from unilateral to bilateral with a caudo-rostral gradient and iii) late appearance of motor deficits when more than 50% of DA neurons have degenerated. The cellular substrates of this process need further investigation in particular of such unilateral to bilateral neurodegeneration. In this study, magnetic resonance spectroscopy imaging (MRSI) was used to map the metabolite distributions at the contro- and ipsilateral side of injection in SN, dorsal striatum, subthalamic nucleus (STN) and mortor cortex in PDC rats at three time points over a period of 120 days following injury. Results will be compared to metabolite distributions obtained in sham-lesioned rats and in a widely used rat model of progressive PD induced by the targeted overexpression in the SN of human α-synuclein mediated by viral vector4. Here we report the preliminary results of the study on brain metabolic profile changes in these models.
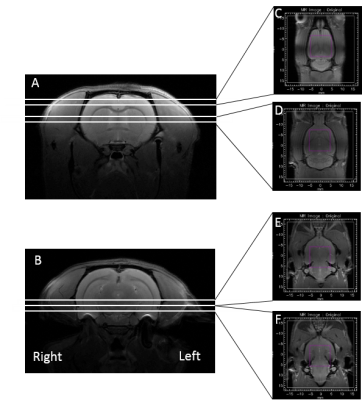
Subjects and Methods: Surgery was performed in 7–8 weeks old rats at the beginning. After anesthesia, animals received unilateral stereotaxic injection of 300 nmol PDC (5 μL of a 60 mM PDC solution; n=7) or vehicle (0.9% NaCl; n=5) in the left SN pars compacta (coordinates in mm versus Interraural Line: AP +2.2, L ±2.0, and DV +3.3). For the α-synuclein model, 1 µL of the recombinant adeno-associated virus vectors (1.5 × 1013 vg/mL) were injected at the same coordinates. Rats were explored 15, 60 and 120 days after surgery. NMR measurements were performed at 11.7 T on a Bruker BioSpec 117/16 Ultra Shielded Refrigerated system. Animals were kept anesthetized with isoflurane and a mixture of air and O2 and secured in a holder. A circular polarized 1H radiofrequency coil was used for excitation associated with a head rat surface coil for signal reception. Breathing and temperature parameters were monitored along the session. The MR protocol included axial and coronal fast spin echo T2-weighted RARE sequences with a field of view of 35x35 mm2; 256x256 data matrix; 1 mm slice thickness; effective TE = 15.72 ms; TR = 2500 ms, RARE factor = 4 and number of averages = 2. 19 axial slices were acquired and allowed positioning for acquisition of the coronal slices as described in figure 1. MRSI acquisition were performed for each region of interest using a CSI sequence with a semi-LASER voxel volume selection (dimensions in mm: FOV 32x32x2, voxel size = 10x10x2, image size = 20x20, resolution = 1.6x1.6x2, TR = 2000ms, TE = 24ms, VAPOR for water suppression). A similar semiLASER-CSI (1 average) was acquired but without water saturation for absolute metabolite quantification. MRSI data were processed under CSIAPO5 in association with a time-domain quantification algorithm based on QUEST6. The metabolite basis set included metabolites and macromolecules. The signals of metabolites will be normalized using water signal intensity as internal reference.
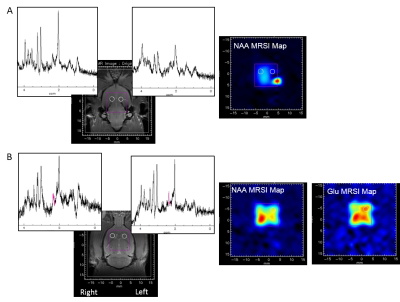
Results: Figure 2A shows the spectra of two voxels localized on the contro and ipsilateral SN on a PDC rat and acquired 60 days post PDC injection. At this time point, an asymmetric NAA repartition was observed on the MRS image in accordance with the dopaminergic denervation induced by PDC injection in the left SN. First results seem also to report higher glutamate levels in the ipsilateral striatum and no change for the other metabolites (figure 2B).
Discussion/Conclusion: These preliminary results showed that 1H MRSI is a useful technique for longitudinal characterization of metabolite profiles in animal models of PD. Completion of this project is expected to provide new information on the commonalities or specificities of the neurochemical changes within key basal ganglia components in two PD models: the well-established α-synuclein overexpression-driven PD rat model, and a new one, the PDC model. Both show progressive DA neuron loss. The comparison of these both models might help identifying substrates involved in the uni- to bilateral progression of neurodegeneration in the PDC model.
Acknowledgements
This work was supported by Saint Jude Grants.References
1. Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson's disease. Mol. Neurobiol. 1996 ;12,73–94. 2. Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS. Neurol. Disord. Drug Targets. 2009 ;8,475–491. 3. Assous M, Had-Aissouni L, Gubellini P, et al. Progressive Parkinsonism by acute dysfunction of excitatory amino acid transporters in the rat substantia nigra. Neurobiol. 2014;65:69-81. 4. Kirik D, Rosenblad C, Burger C, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22(7):2780-91. 5. Le Fur Y, Nicoli F, Guye M, et al. Grid-free interactive and automated data pro- cessing for MR chemical shift imaging data. Magn Reson Mater Phy. 2010;23(1):23–30. 6. Ratiney H, Sdika M, Coenradie Y, et al. Time-domain semi-parametric estimation based on a metabolite basis set. NMR Biomed. 2005;18(1):1–13.Figures