1363
A neuroimaging study of the effects of early vs. late anti-inflammatory treatment in a rodent model of Alzheimer’s disease1Engineering, McGill University, Montreal, QC, Canada, 2McGill University, Montreal, QC, Canada, 3Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montreal, QC, Canada
Synopsis
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with no effective treatments or known biomarkers for definitive diagnosis, substantiating the need for early detection of AD and early intervention. This project employs Magnetic Resonance Spectroscopy (MRS) to measure changes in neurometabolites as compared to behavioural measures of cognitive function, in a transgenic rat model of AD under treatment conditions. Preliminary results suggest that changes in metabolite levels are present before the onset of cognitive impairment, and between treatment and control groups, with some of these changes being sexually dimorphic.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with no effective treatments or known biomarkers for definitive diagnosis, substantiating the need for early detection of AD and early intervention. Pre-clinical development of biomarkers and testing of treatment options in animal models of AD represents an important step towards clinical trials. As such, the general aim of this study is to assess longitudinal changes in neurochemistry related to AD pathology in the TgF344-AD rat model of AD under treatment conditions. This project employs Magnetic Resonance Spectroscopy (MRS) to monitor changes in hippocampal neurotransmitter levels. Previous proton MRS studies have identified reduced levels of N-acetylaspartate (NAA) and glutamate (Glu) (indicative of neuronal loss), and increased levels of myo-inositol (mI) and glutamine (Gln) (indicating gliosis) in rodent models of AD.1–4 Importantly, the neurochemical changes observed in animal models of AD are consistent with changes observed in MRS studies of AD in humans, and the in vivo MRS methods used for studying animal models are fully translatable to human AD subjects.1,5 The progression of these imaging biomarkers was compared against the progression of cognitive deficits, assessed using the Barnes Maze (BM) and Novel Object Placement (NOP) hippocampus-dependent spatial learning and memory tasks. The treatment paradigm consists of early and late interventions with Naproxen, a common non-steroidal anti-inflammatory drug (NSAID), which has been shown to have beneficial effects on disease progression, but only when administered during pre-symptomatic stages of the disease.6–9 Preliminary results suggest that changes in metabolite levels exist before the onset of cognitive impairment and between treatment and control groups, with some of these changes being sexually dimorphic.Methods
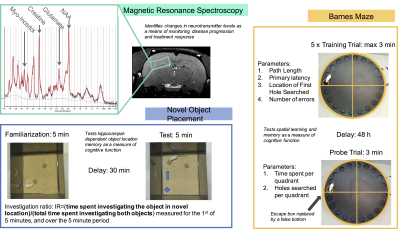
Proton MRS acquisition and analyses: All MRS data acquisitions were performed on a 7 Tesla Bruker Biospec 70/30 scanner. A high-resolution anatomical image was used to guide placement of a region of interest for localized MRS in the dorsal hippocampus (~31 μL). Automated localized shimming was performed on this region using the FASTMAP method (linewidth range 8.8-14.7 Hz).10 MRS measures were acquired from this region using the PRESS sequence. MRS pre-processing was performed using the FID-A processing toolbox.11 All proton MRS spectra were analyzed in LCModel using a basis set simulated using the FID-A toolkit.11 All metabolite concentrations were referenced to total creatine. Novel Object Placement: The NOP test evaluates object location memory of two previously explored objects, one in a familiar location, and one in a novel location. Investigation ratio during the first minute was used to assess object location memory as a measure of cognitive function (data not shown).12 Barnes Maze: A five-trial training protocol with a one-session probe test was used to assess spatial learning and memory. Primary latency, path length, and primary hole searched were recorded during the training trials. Measures of time spent per quadrant and holes searched per quadrant were recorded during the probe trial (data not shown).13Results
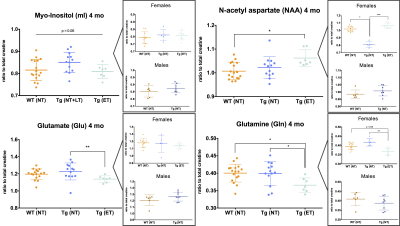
MRS measurements in 4-month-old wildtype no treatment (WT NT), transgenic no treatment (Tg NT), and transgenic early treatment (Tg ET) rats show changes in Glutamate, Glutamine, and NAA levels as a function of disease state and/or treatment response (Figure 2). Each metabolite was additionally analyzed by sex, and sexually dimorphic changes were observed in NAA and Gln, suggesting further analyses continue to be split by sex.Discussion
Proton MRS enables quantitative measurement of the concentrations of up to 20 different metabolites in the brain, many of which are established biomarkers of known pathological traits. For example, in both humans and mouse models of AD, reduced NAA and Glu levels and elevated Gln and mI have been observed, due to the presence of neuronal loss and gliosis, respectively.5,7 We observe significantly decreased NAA in Tg NT females, with this decrease appearing to be mitigated with early Naproxen treatment. This result fits with predicted delayed progression of neurochemical changes in Tg rats treated with Naproxen compared to untreated Tg rats, as has been shown with other NSAIDs.7–9 Other metabolite levels show varying differences between groups and sexes; additional animals and time points will enable more accurate interpretation of these preliminary results. Further analysis of metabolites by sex was an intentional decision due to literature reporting differences in disease presentation in men versus women.Conclusion
These preliminary results suggest that MRS measurements can be used to monitor disease progression and treatment response in an AD rodent model. Together, the neuroimaging paradigm and anti-inflammatory therapeutic intervention represent a promising step towards a better understanding of disease progression, as well as the development of new prevention and treatment strategies.Acknowledgements
No acknowledgement found.References
1. Marjańska M, Curran GL, Wengenack TM, et al. Monitoring disease progression in transgenic mouse models of Alzheimer’s disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci. 2005;102(33):11906-11910. doi:10.1073/pnas.0505513102.
2. Von Kienlin M, Künnecke B, Metzger F, et al. Altered metabolic profile in the frontal cortex of PS2APP transgenic mice, monitored throughout their life span. Neurobiol Dis. 2005;18(1):32-39. doi:10.1016/j.nbd.2004.09.005.
3. Dedeoglu A, Choi JK, Cormier K, Kowall NW, Jenkins BG. Magnetic resonance spectroscopic analysis of Alzheimer’s disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 2004;1012(1-2):60-65. doi:10.1016/j.brainres.2004.02.079.
4. Nilsen LH, Melø TM, Saether O, Witter MP, Sonnewald U. Altered neurochemical profile in the McGill-R-Thy1-APP rat model of Alzheimer’s disease: a longitudinal in vivo 1 H MRS study. J Neurochem. 2012;123(4):532-541. doi:10.1111/jnc.12003.
5. Gao F, Barker PB. Various MRS application tools for Alzheimer disease and mild cognitive impairment. Am J Neuroradiol. 2014;35(6 SUPPL.). doi:10.3174/ajnr.A3944.
6. Jantzen PT, Connor KE, DiCarlo G, et al. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22(6):2246-2254. doi:22/6/2246 [pii].
7. Marjańska M, Weigand SD, Preboske G, et al. Treatment effects in a transgenic mouse model of alzheimer’s disease: A magnetic resonance spectroscopy study after passive immunization. Neuroscience. 2014;259:94-100. doi:10.1016/j.neuroscience.2013.11.052.
8. Choi J-K, Carreras I, Aytan N, et al. The effects of aging, housing and ibuprofen treatment on brain neurochemistry in a triple transgene Alzheimer’s disease mouse model using magnetic resonance spectroscopy and imaging. Brain Res. 2014;1590:85-96. doi:10.1016/j.brainres.2014.09.067.
9. Carreras I, McKee AC, Choi J-K, et al. R-flurbiprofen improves tau, but not Aß pathology in a triple transgenic model of Alzheimer’s disease. Brain Res. 2013;1541:115-127. doi:10.1016/j.brainres.2013.10.025.
10. Gruetter R. Automatic, localized in Vivo adjustment of all first???and second???order shim coils. Magn Reson Med. 1993;29(6):804-811. doi:10.1002/mrm.1910290613.
11. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2017;77(1):23-33. doi:10.1002/mrm.26091.
12. Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113(3):509-519. doi:10.1007/PL00005603.
13. Attar A, Liu T, Chan WTC, et al. A shortened barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PLoS One. 2013;8(11). doi:10.1371/journal.pone.0080355.
Figures