1359
Contribution of Intramyocellular Lipids to the Decrease in Muscle Density with Age1Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, United States, 2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 3Longitudinal Studies Section, National Institute on Aging, National Institutes of Health, Baltimore, MD, United States
Synopsis
Muscle density has been shown to decrease with age. However, the basis for this decrease remains unclear. We hypothesize that this decrease is associated with increased IMCL, and evaluated this relationship using localized 1H MRS of the vastus medialis muscle. We find that increased IMCL and decreased muscle density are strongly correlated across a large age range, even after controlling for multiple potential confounding variables.
INTRODUCTION
Sarcopenia, defined as the degenerative loss of skeletal muscle mass and strength accompanying advancing age, is a marker for limitations in skeletal muscle function.1,2 Age-associated sarcopenia is accompanied by decreased muscle density as quantified by computerized tomography (CT).3 However, the pathophysiologic basis of decreased muscle density has not been established. The replacement of parenchymal muscle tissue by adipose tissue 4,5,6 is associated with diminished performance including frailty 7, decreased lower extremity function 8,9,10 and limitations in mobility, 11,12 increased risk of hip fracture 13, and decreased muscle quality, defined as the force generated by muscle.14 Lower muscle density has also been associated with metabolic derangements including loss of oxidative enzyme capacity.15 These considerations motivate investigation of the contribution of intramyocellular lipids (IMCL) to decreasing muscle density. IMCL represents fat stored in the form of cytoplasmic droplets within myocytes and is associated with impaired energy metabolism.16 It is for the most part uniformly distributed throughout the muscle, and may therefore represent a marker of fat infiltration in muscle.17,18,19 IMCL can be measured by magnetic resonance spectroscopy (MRS); in this work, we explore the role of IMCL accumulation in the decline of muscle density with age in a large sample of healthy individuals across a large age range, drawn from the Baltimore Longitudinal Study on Aging (BLSA).EXPERIMENTAL DESIGN & METHODS
314 subjects were studied: 142 males (mean age 71.42; range 34-92) and 172 females (mean age 70.13; range 32-98). Other characteristics of the study population, including mean values of outcome measures, are shown in Table 1 and reflect the relatively healthy population characteristics of the BLSA. IMCL values were obtained from in vivo 1H spectra acquired using a 3T Philips Achieva MR scanner (Philips, Best, The Netherlands), using the internal body coil for excitation and a quadrature 10 cm flex receive-only coil fastened over the vastus medialis muscle of the left thigh. Localized spectra were obtained using a PRESS sequence, with a voxel size of 8x8x40 mm, 32 signal averages, TE = 55ms, TR = 2 s, and a receiver bandwidth of 2000 Hz. The number of complex pairs of data points in one scan, without zero-filling, was 1024. Both non-water-suppressed and water-suppressed spectra were acquired and processed using LCModel software.20 IMCL peak area was normalized to the unsuppressed water peak area. We ensured the accurate measurement of IMCL by 1H MRS through application of specific quality control criteria, which included assessment of spectra baseline, quality of fit, and peak resolution. Muscle density was measured using computerized tomography (CT; Somatom Sensation 10; Siemens, Malvern, PA) and quantified with BonAlyse software (Jyvaskyla, Finland) following standard techniques.21 We evaluated the correlation between IMCL and muscle density without the confounding factor of age though formal age-adjustment, with results visualized using local polynomial regression (LOESS).22RESULTS
Muscle Density vs. Age
Muscle density was evaluated as a function of age (Figure 1). Initial analyses were performed adjusting for age, sex, race and IMCL. IMCL was found to be a significant covariate (p<0.001). Given the relationship between IMCL and obesity, further analyses were undertaken with adjustments made for BMI, circulating triglyceride concentration and waist circumference (Table 2). Increased waist circumference was found to be significantly correlated with a decrease in muscle attenuation. After adjusting for BMI, circulating triglyceride concentration and waist circumference, IMCL remained as a significant covariate (p<0.01).
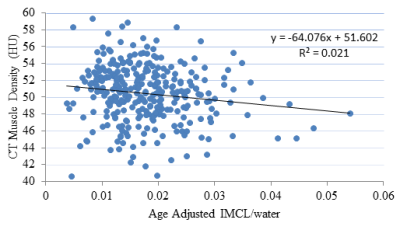
Muscle Density vs. Age-adjusted IMCL
A multivariate analysis was performed to determine if obesity, in general, affected the relationship between age-adjusted IMCL and muscle density (Figure 2). After adjusting for sex, race, BMI, circulating triglyceride concentration and waist circumference, the correlation between muscle density and age-adjusted IMCL remained significant (p<0.05).
DISCUSSION
Muscle density is a crucial correlate in the development of disease and disability, with low density being associated with increased hospitalizations in the aging population.8 Decreased muscle density is also associated with poor physical function, endurance and strength in older adults.4,9 However, the basis for the loss of muscle density with age has not been established. Here, we find that increased IMCL is independently associated with decreased muscle density; although causality cannot be established from our data, the fact that this relationship is maintained after adjustment for multiple confounding factors motivates further investigation of a causal link.CONCLUSIONS
IMCL was significantly associated with the decrease in muscle density seen with advancing age. While this does not establish causality, it renders a causal relationship highly plausible. These results are consistent with but greatly extend previous findings.23Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.
References
1. Kalyani, R.R., M. Corriere, and L. Ferrucci, Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol, 2014. 2(10): p. 819-29.
2. Roubenoff, R. and V.A. Hughes, Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci, 2000. 55(12): p. M716-24.
3. Lauretani, F., et al., Axonal degeneration affects muscle density in older men and women. Neurobiol Aging, 2006. 27(8): p. 1145-54.
4. Goodpaster, B.H., et al., Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985), 2001. 90(6): p. 2157-65.
5. Addison, O., et al., Intermuscular fat: a review of the consequences and causes. Int J Endocrinol, 2014. 2014: p. 309570.
6. Goodpaster, B.H., et al., Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985), 2000. 89(1): p. 104-10.
7. Cesari, M., et al., Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr, 2006. 83(5): p. 1142-8.
8. Cawthon, P.M., et al., Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc, 2009. 57(8): p. 1411-9.
9. Visser, M., et al., Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc, 2002. 50(5): p. 897-904.
10­­. Hicks, G.E., et al., Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci, 2005. 60(7): p. 882-7.
11. Visser, M., et al., Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci, 2005. 60(3): p. 324-33.
12. McDermott, M.M., et al., Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation, 2009. 120(12): p. 1048-55.
13. Lang, T., et al., Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res, 2010. 25(3): p. 513-9.
14. Conroy, M.B., et al., Muscle strength, mass, and quality in older men and women with knee osteoarthritis. Arthritis Care Res (Hoboken), 2012. 64(1): p. 15-21.
15. Schrauwen-Hinderling, V.B., et al., Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia, 2007. 50(1): p. 113-20.
16. Li, Y., et al., Skeletal intramyocellular lipid metabolism and insulin resistance. Biophys Rep, 2015. 1: p. 90-98.
17. Schrauwen-Hinderling, V.B., et al., Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring), 2006. 14(3): p. 357-67.
18. Weis, J., et al., Quantification of intramyocellular lipids in obese subjects using spectroscopic imaging with high spatial resolution. Magn Reson Med, 2007. 57(1): p. 22-8.
19. Brumbaugh, D.E., et al., Intramyocellular lipid is associated with visceral adiposity, markers of insulin resistance, and cardiovascular risk in prepubertal children: the EPOCH study. J Clin Endocrinol Metab, 2012. 97(7): p. E1099-105.
20. Provencher, S.W., Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med, 1993. 30(6): p. 672-9.
21. Moore, A.Z., et al., Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc, 2014. 62(2): p. 230-6.
22. Cleveland, W.S. and S.J. Devlin, Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. Journal of the American Statistical Association, 1988. 83(403): p. 596-610.
23. Larson-Meyer, D.E., et al., Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring), 2006. 14(1): p. 73-87.
Figures
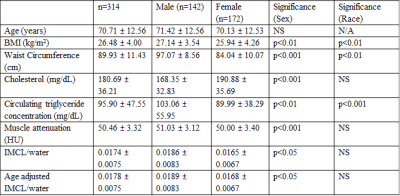
Table 1: Participant Characteristics.
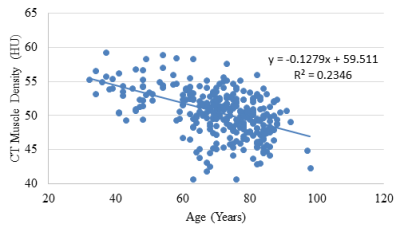
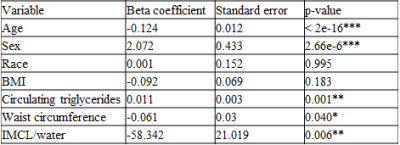
Table 2: Multivariate analysis adjusting for obesity markers in the muscle density and age model. IMCL is a significant covariate in the relationship between muscle density and age, indicating that it may play a significant role in this relationship, independent of age. Significant codes: p<0.001 ***, 0.01 **, 0.05 *.