1358
Higher apparent diffusion coefficients in the older human brain1University of Minnesota, Minneapolis, MN, United States, 2Veterans Affairs Health Care System, Minneapolis, MN, United States
Synopsis
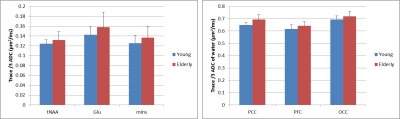
The goal of this study was to compare the apparent diffusion coefficients (ADC) of the five major metabolites between young and older adults. Three brain regions were studied at 3 T using STEAM: prefrontal, posterior cingulate and occipital cortices. This study shows that the diffusivities of total N-acetyl aspartate, glutamate and myo-inositol are higher (7% on average) in the posterior cingulate cortex in older adults while no significant differences in ADC for the five major metabolites are observed in the other two brain regions studied. The ADCs of water are also higher in older adults in all three brain regions.
Purpose
Normal brain aging is associated with changes occurring at all levels e.g., decline in a number of cognitive functions, changes in the volume of the brain, the amount of white matter and iron content1. Using non-invasive techniques such as structural magnetic resonance imaging, it was previously demonstrated that the brain volume changes correlate with age2. In addition, diffusion-weighted imaging (DWI) assessing the brain microstructure showed that the apparent diffusion coefficient (ADC) of water gradually increases from adulthood into the senescence period3. Another promising technique which allows the investigation of the intracellular microenvironment is diffusion-weighted magnetic resonance spectroscopy (DW-MRS). This technique measures the diffusivity of metabolites (e.g., total N-acetyl aspartate (tNAA), total creatine (tCr), total choline (tCho), glutamate (Glu) and myo-inositol (mIns)) such that specific information on compartmentation can be obtained at both cellular and subcellular levels. A previous study reported a decrease in the ADC of tNAA, tCr and tCho in older adults4. However, no change in the ADC of water was found between the young and older cohort and is in contradiction with previous DWI studies. Therefore, the aim of this study was to compare the trace/3 ADC values of the five major metabolites and water in the human brain between young and older adults. A STEAM sequence was used where measurement biases due to cross-terms were avoided by using positive and negative diffusion gradient polarity, as recently demonstrated5.Methods
32 young (21±1 years) and 26 older (74±3 years) adults were scanned on a Siemens 3T scanner after giving informed consent for the study approved by the IRB. Body coil was used for excitation while the 32-channel receive-only head-coil was used for signal reception. ADC data were measured using STEAM (TE/TM/TR=21.22/105/3000 ms) from three VOIs: prefrontal (PFC, 15.6 mL), posterior cingulate (PCC, 15.6 mL) and occipital (OCC, 15.9 mL) cortices. Diffusion weighting was applied using bipolar gradients in three orthogonal directions5. To remove cross-term effects between all applied gradients, additional spectra were acquired with diffusion gradients of opposite polarity for each gradient direction. Data were acquired at two b-values; a null b-value (16 averages) and a high nominal b-value (48 averages) of 3172 s/mm2 in all three regions. Water reference scans at both b-values were also acquired for eddy-current correction. All spectra were processed in MATLAB6: eddy current effect was first corrected, low SNR spectra were removed, and remaining spectra corrected using single-shot frequency and phase algorithms. Spectra, at null b-value and for all six gradient diffusion directions, were summed separately and analyzed with LCModel7 using simulated basis sets and measured macromolecule spectra. ADC for tCr, tCho, tNAA, Glu, mIns and water were calculated.Results and Discussion
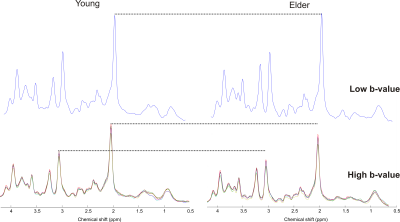
Diffusion-weighted STEAM spectra acquired in the PCC from one young adult and older adult are shown in Figure 1. At low b-value, similar spectral pattern with comparable SNR was observed between the two cohorts. At high b-value even though the diffusion gradients were identical, the spectrum amplitude was lower in older adult than young suggesting higher ADC in the older adult. Indeed, ADC of tNAA was higher in older compared to young adults in PCC: 0.12±0.01 µm2/ms vs. 0.13±0.02 µm2/ms, P=0.04 (Figure 2). Similarly, ADCs of Glu and mIns were higher in older adults by ~10% in PCC (P ≤0.03). However, no significant difference in ADC values was observed for the 5 major metabolites in the other two regions, i.e., PFC and OCC. The fact that PCC is more sensitive to biochemical changes agrees with our recent work where significant neurochemical changes were observed in PCC compared to OCC8. The trace/3 ADC of water was also found to increase by 3.6-6.8% in older adults in all three brain regions with the largest difference observed in PCC (Figure 2). This increase in ADC is in agreement with literature values obtained previously using DWI3. The ADC values reported in the current study are in disagreement with another study4 where a decrease of at least 25% was observed for the 3 main singlets. This discrepancy might be related to difference in the brain region studied, echo-time, diffusion times, and cross-term effects.Conclusion
This study shows that the diffusivity of tNAA, Glu and mIns is higher in the posterior cingulate cortex during senescence while no changes in ADC of the five major metabolites were observed in the prefrontal cortex and occipital cortex. Interestingly, the ADC of water was also higher in the brain of older adults in all three brain regions.Acknowledgements
This work was supported by NIH grants: R21AG045606, P41 EB015894, P30 NS076408. We thank Andrew Oliver for study coordination and for neuropsychological testing, Sarah Bedell for study coordination and Akshay Patke for neuropsychological testing.
References
1. Peters. Ageing and the brain. Postgrad Med J. 2006;82(964):84-8.
2. Walhovd et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011; 32(5):916-32.
3. Watanabe et al. Age-related apparent diffusion coefficient changes in the normal brain. Radiology 2013; 266(2):575-82.
4. Zheng et al.The Effect of Age and Cerebral Ischemia onDiffusion-Weighted Proton MR Spectroscopy ofthe Human Brain. AJNR Am J Neuroradiol. 2012;33(3):563-8.
5. Deelchand et al. Apparent diffusion coefficients of the five major metabolites measured in the human brain in vivo at 3T. Magn. Reson. Med. doi: 10.1002/mrm.26969.
6. Deelchand. MRspa (https://www.cmrr.umn.edu/downloads/mrspa/)
7. Provencher. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672-9.
8. Marjańska et al. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1H magnetic resonance spectroscopy at 7 T. Neuroscience. 2017 Jun 23;354:168-177
Figures