1357
Anterior cingulate cortex glutathione decreases with age - faster in women than in men?1Neuroscience and Psychiatry Unit, Division of Neuroscience and Experimental Psychology, University of Manchester, Manchester, United Kingdom, 2Division of Informatics, Imaging and Data Sciences, University of Manchester, Manchester, United Kingdom
Synopsis
The anti-oxidant glutathione (GSH) may protect against ageing. Significantly lower GSH in the occipital cortex has been reported in elderly compared to young healthy volunteers. Here we show that GSH is also decreased in middle-aged (N=8, 39-54y) compared to young (N=8, 22-32y) healthy subjects in the anterior cingulate but not the occipital cortex using GSH-edited MEGA-PRESS at 3T. This significant difference is driven by the women in the middle-age sub-group (significantly lower GSH than in men). This suggests that age-related oxidative stress begins earlier in women compared to men and sex composition of a studied group could influence results.
Introduction
Oxidative stress has been associated with normal ageing and several neurodegenerative diseases1. Glutathione (GSH) plays an important role as an anti-oxidant in the protection against oxidative stress1,2 and, by consequence, it could be protective against ageing. Because of its low concentration and superposition with other metabolite spectral signatures, GSH concentration is difficult to be estimated from short echo-time spectra and it has been suggested that MEGA-PRESS enables more reliable measurement of GSH in-vivo compared to PRESS at 3T3. Significantly lower GSH has been reported at 4T in the occipital cortex (OCC) of elderly compared to young healthy volunteers4. However, there is no inference whether the sex composition of the groups might have an effect on the results. Additionally, it is currently unknown whether GSH is decreased by middle-age.
This study tested the hypothesis that GSH would already be decreased in middle-age healthy controls compared to young controls. We also investigated whether the sex composition of the group had any effect on results.
Methods
After having obtained written informed consent, spectra were acquired from 16 healthy controls [comprising 1F 7M young (22-32 y) and 4F 4M middle-age (39-54 y) subjects] using a 3T Philips Achieva and a 32 channel head coil. A 3.5 x 4.0 x 2.0 cm3 and a 3 x 3 x 3 cm3 VOIs were placed in the anterior cingulate cortex (ACC) and OCC, respectively, and MEGA-PRESS spectra were acquired (TE/TR = 130/2000 ms, 64 dynamics, NSA4, acquisition time: 8.5 minutes).
Spectra were processed and fitted using jMRUI5 (v6.0-alpha) software and AMARES6 to measure the edited GSH peak centered at 2.95ppm. Unsuppressed water was used as internal reference. T1-weighted images were segmented to obtain the percentages of gray matter, white matter and cerebrospinal fluid (CSF) in each spectroscopy VOI. GSH concentrations were corrected for differences in CSF percentages, assuming negligible GSH content in CSF.
Because age was non-normally distributed, Spearman`s correlation coefficient, ρ, was used to investigate the correlation between GSH and age within the entire group (N=16). The GSH estimations for the two age sub-groups (young vs. middle-age) were compared and significant differences were assessed for each VOI using the one-tailed unpaired t-test, testing the prior hypothesis that GSH is reduced in middle-age. Significant differences between females and males within the middle-age sub-group were tested using the two-tailed unpaired t-test.
Results
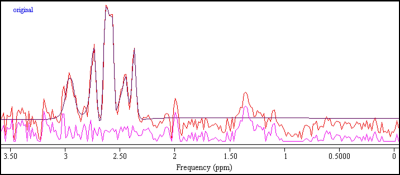
An example of an edited-spectrum fit is shown in Fig 1. In ACC, GSH concentrations (mean±SD) for the young and middle-age sub-groups were 3.84±0.79 mM and 3.03±1.03 mM respectively. In OCC, they were 4.07±1.34 mM in the young and 3.16±1.22 mM in the middle-age. The mean±SD Cramer-Rao Lower Bounds (CRLB), in percentages, were 12.4±6.7 % in ACC and 16.0±9.2 % in OCC.
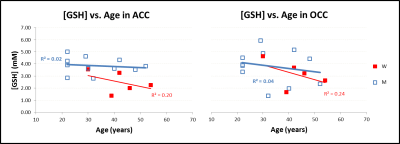
When including all healthy volunteers, a moderate negative correlation (-0.448, ρ<0.05) was found between GSH and age in ACC, but not in OCC. Additionally, GSH was significantly lower in the middle-age compared to the young sub-group in ACC (p=0.05), but not in OCC. Within the middle-age sub-group, GSH concentrations were significantly lower in women compared to men (p<0.05) in ACC, but not in OCC.
Fig 2, where GSH concentrations for men and women in ACC and OCC are plotted versus age, indicates in both voxels that the age-related decline in GSH is largely confined to the women in both regions with a minimal decline observed in men.
Discussion and conclusions
In the present study, MEGA-PRESS was sensitive enough to detect significant differences in GSH between various sub-groups, confirming its suitability for measuring GSH in vivo. In ACC, GSH was significantly decreased in the middle-age compared to the young sub-group and it seems that the significant difference is driven by the women in the middle-age sub-group, which suggests that age-related oxidative stress begins earlier in women compared to men. It is therefore important to note that the sex composition of a studied group could influence the GSH concentrations and presence or absence of significant differences between sub-groups and/or time-points. Additionally, the results show that, besides OCC, ACC could also be an interesting brain region for investigating metabolic changes in healthy ageing in men and women.Acknowledgements
UK-Medical Research Council; European Commission-TRANSACT-FP7; NIHR/WT Clinical Research Facility for support of scanning.References
1. Choi I-Y, Lee P, Denney DR et al., Dairy intake is associated with brain glutathione concentration in older adults. Am.J.Clin.Nutr. 2015; 101:287-293.
2. Rae CD, Williams SR., Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Analytical Biochemistry 2017; 529: 127-143.
3. Sanaei Nezhad F, Anton A, Parkes LM et al., Quantification of glutathione in the human brain by MR spectroscopy at 3Tesla: Comparison of PRESS and MEGA-PRESS. Magn.Res.Med. 2017; 78(4):1257-1266.
4. Emir UE, Raatz S, McPherson S. et al., Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011; 24: 888–894.
5. Stefan D, Di Cesare F, Andrasescu A et al., Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas. Sci. Tech. 2009; 20.
6. Vanhamme L, van den Boogaart A, Van Huffel S, Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 1997; 129(1): 35–43.
Figures