1354
A 1H/31P MRS study of ATP and GABA modulation induced by anodal transcranial direct current stimulation in primary motor cortex of healthy subjects1Division of Clinical Cognitive Sciences, Department of Neurology, RWTH Aachen University Hospital, Aachen, Germany, 2Institute of Neuroscience and Medicine - 4, Forschungszentrum Jülich GmbH, 52425, Juelich, Germany, 3Faculty of Medicine, Department of Neurology, RWTH Aachen University, JARA, Aachen, Germany
Synopsis
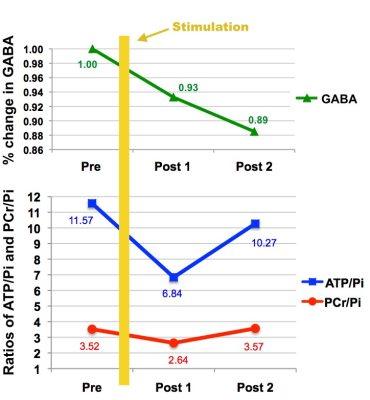
Transcranial direct current stimulation (tDCS) modulates cerebral energy and cortical inhibition. In this study we investigated long-term effects of anodal stimulation on inhibitory neurotransmitter and energy phosphate concentration using proton and phosphorous magnetic resonance spectroscopy. Our results indicate immediate GABA reduction following anodal tDCS and further maintaining the decreased state until the end of the experiment. ATP/Pi and PCr/Pi show initial reduction following anodal tDCS and further sign of recovery by the end of the experiment.
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive technique that modulates excitability of neurons in the cerebral cortex. Anodal tDCS has been reported to modulate gamma-aminobutyric acid (GABA) concentration1 and cerebral energy consumption in motor cortex2. To date very little is known about the duration of tDCS induced effects on cortical inhibition and related energy consumption. This study aims to investigate long-term GABA and energy modulation following anodal tDCS on a primary motor cortex (M1). Proton (1H) and phosphorus (31P) magnetic resonance spectroscopy (MRS) were alternatively employed to measure relative GABA concentrations and energy phosphates, respectively, in M1 up to approximately 75 minutes after stimulation.Materials and Methods
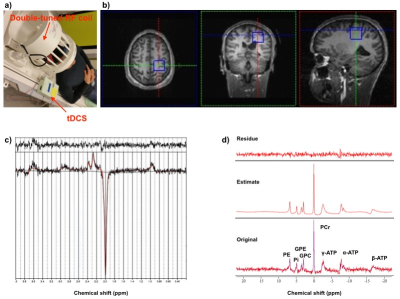
One right-handed healthy volunteer was recruited in RWTH Aachen University. All MR experiments were performed on a 3T Siemens PRISMA whole-body scanner using a commercial double-tuned 1H/31P head coil. Figure 1 summarises the measurement protocol for this study. One set of MRS scans including 1H GABA and 31P sequences and two sets of the scans were carried out before and after the stimulation, respectively. 1 mA anodal and sham tDCS were applied for 10 minutes (Figure 2a) with the anodal electrode placed over the hand area of left M1 and cathodal electrode placed on the right supra-orbital area. GABA-edited 1H and 31P spectra were independently acquired from a volume-of-interest (VOI) of 3 x 3 x 3 cm3 carefully localised on the hand area of the left M1 (Figure 2b). MEGA-PRESS J-editing3 was used for GABA detection (TR/TE = 5350/68 ms and 96 averages), and 3D CSI (TR/TE = 3730/2.3 ms and 6 averages) with NOE enhancement (Waltz4) was utilised for 31P acquisition. Data processing and quantification of GABA and 31P spectra were performed with LCModel4 and jMRUI5 software, respectively.Results
Figure 2c and 2d are examples of a 1H GABA and a 31P spectrum obtained from the motor cortex of the healthy volunteer showing residual, fitting and original data. The GABA signal depicted in Fig. 3 (top) decreased immediately after the anodal stimulation and continued decreasing over the whole planned test measurements. It was found that both ATP/Pi and PCr/Pi ratios reduced after the stimulation but recovered to their original values, which was observed by the Post 2 measurement shown in Fig. 3 (bottom).Discussion and Conclusions
Decrease in GABA concentration corresponds not only to the reported behavioural effects of tDCS stimulation, such as increased motor learning capacity, but also to the timeline of increased brain energy consumption following anodal tDCS as measured with 31P spectroscopy2. Our preliminary results are relevant for the understanding of the mechanism of motor cortical plasticity and metabolic disorders in humans. In the future, we intend to employ a large number of subjects for further quantification and statistical analysis.Acknowledgements
No acknowledgement found.References
1. Nitsche MA, Paulus W., et al. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639.
2. Binkofski F, Loebig M, Jauch-Chara K, Bergmann S, Melchert UH, Scholand-Engler HG, Schweiger U, Pellerin L, Oltmanns KM (2011) Brain energy consumption induced by electrical stimulation promotes systemic glucose uptake. Biological psychiatry 70:690-695.
3. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998;11(6):266–272.
4. Provencher SW, MRM, 30:672, 1993, Estimation of metabolite concentrations from localised in vivo proton NMR spectra.
5. Vanhamme L et al., JMR, 129: 35, 1997, Improved Method for Accurate and Efficient Quantification of MRS data with use of prior knowledge.
Figures