1351
Detection of acute changes in glutamate with MR Spectroscopy using an N-acetylcysteine challenge1University Children's Hospital, Zurich, Switzerland, 2University Hospital, Zurich, Switzerland, 3GE Healthcare, Potsdam, Germany
Synopsis
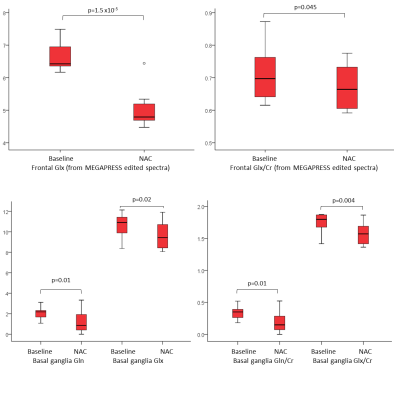
We examined acute changes in MRS-visible glutamate and glutamine after stimulation with N-acetylcysteine (NAC), since NAC reportedly decreases synaptic glutamate via activation of inhibitory metabotropic glutamate receptors. In 10 healthy adults, NAC significantly reduced Glx in the basal ganglia and prefrontal cortex. In the basal ganglia, the changes in Glx were driven by changes in Gln, suggesting that Gln might represent a proxy marker for synaptic glutamate. In the frontal lobe, the MEGAPRESS edited spectra showed greater sensitivity to changes in Glx than short TE PRESS or the edit OFF subspectra. Acute compartmental shifts in glutamate are detectable with MRS.
Introduction
Glutamate is the primary excitatory neurotransmitter and one of the most abundant metabolites in the human brain. Glutamatergic neurotransmission is critical for healthy brain function, and alterations in glutamate signalling are thought to be central to a number of brain disorders including epilepsy, Alzheimer’s disease, and autism.1-3 Within the brain, glutamate is present in millimolar concentrations in mitochondrial, cytosolic, and vesicular compartments within a larger neuronal pool and a smaller astrocytic pool.4 Vesicular glutamate is released into the synaptic cleft during neurotransmission and is rapidly taken up by astrocytes, where it is converted into glutamine and released back into the synaptic cleft to be taken up by neurons and cycled back into glutamate. Nonvesicular free glutamate is released from astrocytes into the extracellular fluid via the cysteine-glutamate antiporter.5 While this extracellular glutamate is present at much lower (eg micromolar) concentrations, it can stimulate inhibitory metabotropic glutamate receptors on glutamatergic neurons, thereby causing a reduction in synaptic glutamate release. This mechanism is thought to underly the apparent increase in extracellular glutamate (and corresponding decrease in synaptic glutamate) observed with administration of N-acetylcysteine.6,7 Given the rapid uptake of glutamate from the synaptic cleft and the extracellular space by astrocytes, the MRS-visible glutamate signal is largely ascribed to the intracellular cytoplasmic compartments,4 but the exact origin of the MRS glutamate signal remains unknown. However, since the cerebral concentration of glutamine is tightly coupled to that of neurotransmitter glutamate via the glutamate-glutamine cycle some authors have suggested that glutamine might represent a proxy marker for synaptic glutamate.8 In this study we use the glutamate (Glu), glutamine (Gln), and combined Glu+Gln (Glx) signals from PRESS and MEGAPRESS 1H MRS to examine acute changes in synaptic glutamate after stimulation with N-acetyl cysteine.Methods
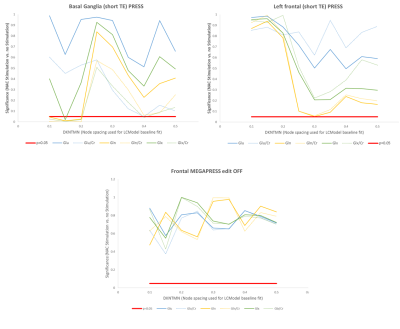
Ten healthy young adult male volunteers were imaged on two scanning sessions on consecutive days, using a GE 3T SIGNA PET/MR scanner. 5g NAC was administered as a continuous infusion one hour prior to one of the scan sessions, and the order of sessions with and without prior NAC administration was counterbalanced across subjects. Single voxel 1H spectra were collected from voxels within the basal ganglia (25x25x25 mm3) and left prefrontal cortex (20x30x40 mm3) with PRESS (TE/TR=35/3000 ms, 160 averages), and GABA-edited spectra were acquired from the same prefrontal voxel with MEGAPRESS (TE/TR=68/1800 ms, 160 edit ON/OFF pairs). The acquisition time for each (PRESS and MEGAPRESS spectrum) was approximately ten minutes. Spectra were processed with LCModel and Glu, Gln, and Glx levels were quantified both as water-scaled concentrations (corrected for partial volume CSF contamination) and as ratios to Creatine. For the MEGAPRESS data the LCModel fit was performed using the parameter “mega-press-2” to constrain the baseline. For the PRESS (short TE and edit OFF MEGAPRESS) data, the dependence of the metabolite levels on the baseline was examined by varying the parameter DKNTMN, which controls the node spacing of the spline function used to fit the baseline, from 0.1 to 0.5 in steps of 0.05.Results
NAC administration was associated with a significant reduction in Glx in both regions (p<0.05, figure 1). In the frontal lobe, the strongest differences were seen in the Glx concentrations from the MEGAPRESS edited spectra (p=1.5x10-5). Trend-level changes in Gln and Gln/Cr were observed in the short TE PRESS spectra, but only for dkntmn=0.3 (figure 2). No significant changes in Glx, Glu, or Gln were seen in the edit OFF subspectra. In the basal ganglia, significant differences in Glx, Gln, Glx/Cr, and Gln/Cr were detected, but only for certain values of dkntmn (figure 2).Discussion
Acute changes in glutamate with NAC are detectable with 1H MRS, both in the basal ganglia and prefrontal cortex. In the short TE PRESS spectra, this change in Glx was driven by a change in Gln rather than a change in Glu, lending weight to the notion that Gln can provide a proxy marker for neurotransmitter/synaptic glutamate. However, the observed changes in Gln and Glx in the short TE spectra were variable and showed a strong dependence on the node spacing used for the LCModel baseline fit (figure 2). In the MEGAPRESS data, the Glx signal from the subtraction spectrum showed a consistent and highly significant decrease with NAC administration, but the Glx signal from the edit OFF lines did not alter significantly, and no significant changes in Gln or Glu were observed individually.Conclusion
The MRS-visible Glx and Gln signals are sensitive to acute compartmental shifts in glutamate. The MEGAPRESS subtraction spectrum appears to show high sensitivity to changes in Glx with NAC administration.Acknowledgements
This study was supported by a research grant from the Hartmann-Mueller Foundation.References
1. Drenthen GS, et al. Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Res 2016;256:44-49
2. Riese F, et al. Posterior cingulate γ-aminobutyric acid and glutamate/glutamine are reduced in amnestic mild cognitive impairment and are unrelated to amyloid deposition and apolipoprotein E genotype. Neurobiol Aging. 2015;36(1):53-9
3. Chowdhury FA, et al. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J Magn Reson Imaging. 2015;41(3):694-9
4. Maddock RJ & Buonocore MH. MR Spectroscopic Studies of the Brain in Psychiatric Disorders. Curr Topics Behav Neurosci 2012: DOI: 10.1007/7854_2011_197
5. Baker DA, et al. The Origin and Neuronal Function of In Vivo Nonsynaptic Glutamate. The Journal of Neuroscience, October 15, 2002, 22(20):9134–9141
6. Moran MM, et al. Cystine/Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking. J Neurosci. 2005; 25(27):6389–6393
7. Dean O, et al. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 2011;36(2):78-86
8. Hancu I & Port J. The case of the missing glutamine. NMR Biomed. 2011;24(5):529-35
Figures