1350
Multi-channel signal combination algorithms for polyunsaturated fatty acids (PUFA) using multiple quantum coherence (MQC) MRS in breast cancer1University of Aberdeen, Aberdeen, United Kingdom, 2Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 3School of Medicine, University of Aberdeen, Aberdeen, United Kingdom, 4Pathology Department, Aberdeen Royal Infirmary, Aberdeen, United Kingdom
Synopsis
Polyunsaturated fatty acid (PUFA) is associated with malignant transformation of breast cancer and can be extracted from overwhelming background signals using multiple quantum coherence (MQC) MRS. Since MQC loses half of the signal, SNR enhancement through effective combination of signals acquired from multi channel coils holds significant potential. Investigations so far focused on conventional brain MRS, with drastically different metabolites and cluttered appearance compared to MQC MRS in breast. We therefore acquired PUFA spectra from 17 fresh breast tumour specimens and a patient on a clinical 3T scanner, and current algorithms of adaptively optimised combination (AOC), S/N2, S/N, Signal evaluated.
Introduction
Polyunsaturated fatty acid (PUFA) plays a major role in the malignant transformation of breast cancer1. However, PUFA spectral peak overlaps with monounsaturated fatty acid (MUFA) and water in conventional magnetic resonance spectroscopy (MRS). Multiple quantum coherence (MQC) MRS eliminates MUFA and water signal effectively at the expense of sacrificing half of the signal2. Effective combination of conventional MRS signal from multi channel coils has been proposed to increase the signal to noise ratio (SNR) without additional hardware in brain MRS. However, female breast has drastically different biochemistry compared to brain, while conventional MRS has a cluttered spectral appearance compared to single peak spectra from MQC MRS. We therefore set out to evaluate current channel combination methods on improving SNR of PUFA spectra collected using MQC MRS from whole breast tumour specimens and a breast cancer patient.Methods
For ex vivo experiments, seventeen specimens, freshly excised from female patients with invasive ductal carcinoma undergoing mastectomy or wide local excision surgery, were scanned. For in vivo experiments, a female patient with invasive ductal carcinoma participated in the study. The study was approved by the NHS Research Ethics Committee, and written informed consent was obtained from each patient prior to the study.
Data Acquisition: Single voxel MRS data were acquired on a 3T MRI scanner (Achieva TX, Phillips Healthcare, Best, Netherlands), using body coil for uniform transmission. For ex vivo experiments, PUFA spectrum was acquired using a 32-channel receiver coil and MQC PRESS sequence with TR/TE of 1250/130 ms, 1024 data points, spectral bandwidth of 2000 Hz, spectral editing frequency at 2.8 ppm, 256 averages. Reference spectra without water suppression were acquired using single voxel PRESS sequence with TR/TE of 1250/144 ms, 1024 data points, spectral bandwidth of 2000 Hz, 16 averages. The voxel was positioned snug fit to the tumour. For in vivo experiments, spectra were collected from the tumour and contralateral healthy breast in a patient, using a 16-channel receiver coil and identical protocol. The PUFA acquisition in the healthy breast was adjusted with voxel size of 20x20x20 mm2 and 128 averages.
Data Processing: Raw data from each channel were processed using in house software written in MATLAB (MathWorks, Natick, MA, USA). The candidate channel combination algorithms were adaptively optimised combination (AOC)3,4, S/N2 weighting5, S/N weighting6,7, Signal weighting8,9 and Equal weighting. The time domain signal of each channel in PUFA data were averaged across the number of averages, and subsequently aligned in phase. Phase shift and channel sensitivity were derived from the phase and amplitude respectively, of the dominant peak in the reference spectrum. The dominant peaks were water (4.7 ppm) for voxels containing tumour and lipid (1.3 ppm) for voxels containing healthy tissue. A weighting factor was then applied to the signal from each channel, and the combined output spectrum computed as the summation of all the weighted signals. The SNR of combined spectrum was defined as the ratio between PUFA amplitude and standard deviation of the real spectrum within the range of 9-12 ppm. The SNR improvement was calculated as percentage increase of SNR normalised to the Equal weighting algorithm. PUFA concentration was quantified using AMARES algorithm in jMRUI and referenced to total lipids in reference spectrum.
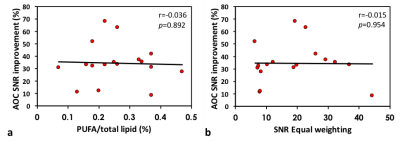
Statistical Analysis: For ex vivo experiments, paired t-tests were performed to identify performance difference in the SNR and SNR improvement among the candidate approaches. Pearson’s correlation tests were carried out to examine the relationship between the SNR improvement derived from AOC algorithm against the baseline SNR of Equal weighting and against PUFA concentration.
Results
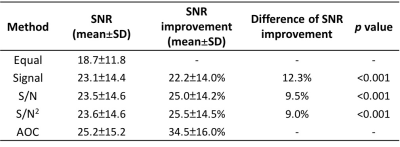
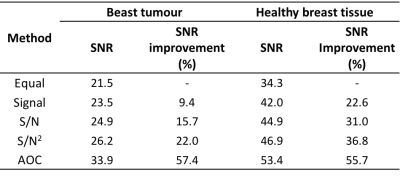
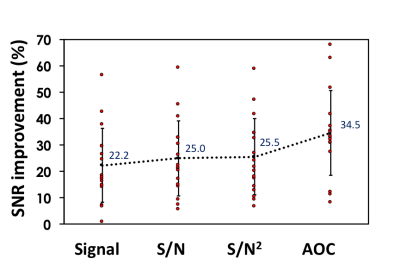
There were significant increases in both SNR (25.2±15.2) and SNR improvement (34.5%±16.0%) from AOC algorithm compared to the other approaches (Table 1, Figure 1). There was no significant correlation between the SNR improvement from AOC algorithm against PUFA concentration (r=-0.308, p=0.229) or against the baseline SNR (r=-0.015, p= 0.954) (Figure 2). The in vivo experiments demonstrated that AOC method provided the maximum SNR improvement compared to the other algorithms, with a 57.4% improvement in the tumour and 55.7% in the healthy tissue (Table 2, Figure 3).Discussion
In this study, four channel combination methods were evaluated for improving SNR on PUFA spectra collected using MQC MRS in breast. AOC approach provided maximal SNR improvement compared to the other channel combination methods, demonstrating its utility on MQC MRS in breast cancer. The performance of AOC was not dependent on the baseline SNR or metabolite concentration under typical clinical experimental condition. Through improved SNR, AOC is the most suitable current algorithm to improve the quantification accuracy of PUFA in a clinical setting.Acknowledgements
The authors would like to thank Dr Mathew Clemence for clinical scientist support as well as Mr Roger Bourne and Ms Mairi Fuller for providing access to the patients. This project was funded by Friends of ANCHOR, and Vasiliki Mallikourti is supported by Tenovus Scotland PhD studentship.References
1. Ge Y, Chen Z, Kang ZB, et al. Effects of adenoviral gene transfer of C. elegans n-3 fatty acid desaturase on the lipid profile and growth of human breast cancer cells. Anticancer Res. 2002;22(2A):537–43.
2. He Q, Shkarin P, Hooley RJ, et al. In vivo MR spectroscopic imaging of polyunsaturated fatty acids (PUFA) in healthy and cancerous breast tissues by selective multiple-quantum coherence transfer (Sel-MQC): A preliminary study. Magn Reson Med. 2007;58(6):1079–85.
3. Fang L, Wu M, Ke H, et al. Adaptively optimized combination (AOC) of magnetic resonance spectroscopy data from phased array coils. Magn Reson Med. 2015;75(6):2235–44.
4. Wu M, Fang L, Ray CE Jr., et al. Adaptively Optimized Combination (AOC) of Phased-Array MR Spectroscopy Data in the Presence of Correlated Noise: Compared with Noise-Decorrelated or Whitened Methods. Magn Reson Med. 2016;30:672–12.
5. Hall EL, Stephenson MC, Price D, et al. Methodology for improved detection of low concentration metabolites in MRS: Optimised combination of signals from multi-element coil arrays. NeuroImage. 2014;86:35–42.
6. Dong Z, Peterson B. The rapid and automatic combination of proton MRSI data using multi-channel coils without water suppression. Magnetic Resonance Imaging. 2007;25(8):1148–54.
7. Avdievich NI, Pan JW, Baehring JM, et al. Short echo spectroscopic imaging of the human brain at 7T using transceiver arrays. Magn Reson Med. 2009;62(1):17–25.
8. Brown MA. Time-domain combination of MR spectroscopy data acquired using phased-array coils. Magn Reson Med. 2004;52(5):1207–13.
9. Natt O, Bezkorovaynyy V, Michaelis T, et al. Use of phased array coils for a determination of absolute metabolite concentrations. Magn Reson Med. 2004;53(1):3–8.
Figures