1348
[Asp], [Glu] and [NAA] changes following traumatic brain injury revealed by J-edited 1H MRS.1Semenov Institute of Chemical Physics, Russian Academy of Sciences, Moscow, Russian Federation, 2Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 3Emanuel Institute of Biochemical Physics, Russian Academy of Sciences, Moscow, Russian Federation
Synopsis
For the first time new method based on MEGA-PRESS pulse sequence for simultaneous aspartate (Asp), glutamate (Glu) and N acetyl aspartate (NAA) cerebral in vivo concentrations quantification were used for monitoring important metabolic changes after severe traumatic brain injury. Revealed Glutamate and Aspartate decrease is associated with excititoxicity (rapidly release of Glu and Asp from vesicles). In addition, Asp reduction might result from reduced availability of Glu.[NAA], marker of neuronal activity, reduction may be associated with synthesis disruption due to reduction of major NAA precussor (Asp).
Introduction
Main problem of 1H MRS is low spectral resolution due to the low magnetic field (<= 3T) as well as low metabolite concentrations and similarity of their chemical structures. Many resonances of different metabolites are overlapped with strong singlets as well as with each other in the spectra of brain tissue. The most common and effective method for solving this problem is a spectral-editing technique using MEGA-PRESS pulse sequence [1]. This method is based on the effect of the refocusing of J-coupling evolution, using frequency-selective RF pulses. In our previous work [2] we represented modification of MEGA-PRESS for direct aspartate quantification. Apart from Asp signal (at 2.65 and 2.80 ppm) spectra obtained contain strong combined Glu and Gln signal at 2.13 ppm. According to [3] contribution of Glu in total signal is about 80-90% (due to the difference between J coupling constants Glu and Gln). Thus, main aim of this study was simulteneously meaure cerebral [Asp], [Glu] and [NAA] after severe traumatic brain injury (sTBI) in acute phase. This is very important task since Asp is main precursor of NAA. It is disturbances in NAA synthetic that can result in [NAA] decrease in different pathologies.
Methods
In this study two groups of participants were enrolled: 8 patients with acute severe TBI (patient’s group: mean age = 13.2±1.8, mean time between MRI examination and injury - 23±4 hours) and 11healthy volunteers (normal group: mean age = 14.4±1.7). The patients were examined under general anesthesia.
Research protocol included MEGA-PRESS spectrum (TE/TR=115ms/1800ms, 40ms editing pulses applied at 3.98 and 5.21 ppm, NSA=8, 48 averages) and PRESS spectrum with the same settings of the transmitting RF system and the shimming system, as well as registration parameters (TE/TR=115ms/1800ms s NSA = 64). For additional outer VOI water suppression spatial saturating pulses were used (REST slabs). Both VOI in size of 25×25×30 were located in the frontal lobe (Fig.1). All investigations were performed on a Phillips 3.0T Achieva TX MRI scanner (Phillips Healthcare, the Netherlands) using an 8-channel “SENSE” receive head coil.
The ratios of metabolite intensities (tNAA, tCr, tCho) to unsuppressed water signal were estimated from PRESS spectrum by LC-model. Absolute concentrations were estimated with corrections on WM, GM and CSF contamination as well as T1 and T2 relaxation. Asp/Cr and Glu/Cr ratios were quantified from MEGA-PRESS spectrum in jMRui 5.2 routine with AMARES algorithm. [Asp] and [Glu] were calculated by multiplying [tCr] by corresponding Asp/Cr and Glu/Cr ratio. [Asp] and [Glu] were expressed in institutional units, since T1 and T2 values as well as J-coupling coefficeints are unknown.
Statistical analysis was performed in STATISTICA 12 using Mann-Whitney U-criterion to evaluate statistical significance of differences between groups. Threshold of significance was set at p<.01.
Results
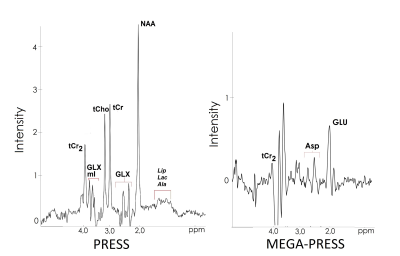
Typical ASP MEGA-PRESS and PRESS spectra are shown on Fig.2. Intergroup analysis of the data obtained from the normal MRI brain area showed a significant simultaneous decrease of [tNAA] (Z=3.58, p<.001), [Glu] (Z=2.51, p<.01) and [Asp] (Z=3.66, p<.001) in patients with acute sTBI as compared to control group (Fig. 3). In this study there was not any significant changes in [tCho] (Z=1.65, p=n.s.). [tCr] was also significant reduced after sTBI (Z=3.12, p<.001).
Discussion
First time we simultaneously measure [Asp], [Glu] and [NAA] in the acute severe TBI. Our observation of a decrease in Glu is consistent with Glu release from neurons [4].Traumatic brain injury induces a rapid cascade of neuronal depolarization and release of the excitatory neurotransmitter Glu. The resulting overstimulation of glutamate receptors initiates excitotoxic mechanisms culminating in neuronal damage (excitotoxicity). Since Asp is also excitatory neurotransmitter, the same process of Asp excitotoxicity may occur, that can result in [Asp] reduction revealed. The lower Asp observed might also result from reduced availability of Glu, which is depleted rapidly from the neuron after TBI. Such strong reduction of Asp obtained in our study (56%) is likely result from both synthesis reduction as well as Asp excitotoxicity.
The main amount of NAA is synthesized in the mitochondria of neurons from Asp and AcCoA by L-aspartate N-acetyltransferase (AspNAT). Reduced [NAA] may be caused by reduction of Asp – one of two main precursors. Further NAA reduction reflects substantial focal neuronal loss and can correlate with injury severity and functional outcome [5]. Decrease in [tCr] concentration may indicate increased energy costs to combat the consequences of injury [6].
Conclusion
Monitoring of changes in NAA and Glu concentrations in vivo after TBI is very important task since in vivo NAA levels as well as CSF Glu levels has strong correlations with functional outcome [7] and severity of brain tissue damage [5] respectively. Asp role is also important since it takes major role in the synthesis on NAA.Acknowledgements
This work was supported by RFBR Grant 17-04-01149 AReferences
[1] Mescher M., Merkle H. et al. Simultaneous in vivo spectral editing and water suppression. NMRBiomed. 1998;11:266-272
[2] Menshchikov P., Semenova N., Akhadov T. Quantification of cerebral aspartate concentration in vivo using proton magnetic resonance spectroscopy. Bulletin of the Lebedev Physics Institute. 2017;44(3):56-60.
[3] P. Menshchikov, T. Akhadov, N. Semenova. MEGA-PRESS for simultaneous aspartate and glutamate quantification at 3T Magn Reson Mater Phy 2017;27:5499
[4] Bartnik B., Sutton R., Fukushima M et al SMUpregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury J Neurotrauma. 2005;22:1052–1065
[5] Garnett M., Blamire A., Styles B. et al Brain. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury 2000;123:1403–1409
[6] Harris J., Yeh H., Lee P. et al. Altered neurochemical profile after traumatic brain injury: 1H-MRS biomarkers ofpathological mechanisms J Cereb Blood Flow Metab. 2012;32(12):2122–2134.
[7] Farooqui A. 2008 Neurochemical Aspects of Excitotoxicity pp 14-20