1346
Tracking changes in glutamate using dynamic MRS in response to an acutely painful stimulus.1Kinesiology, University of British Colombia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries (ICORD), Vancouver, BC, Canada, 3Radiology, University of British Colombia, Vancouver, BC, Canada, 4ImageTech Lab, SFU, Simon Fraser University, Vancouver, BC, Canada, 5Philips Healthcare Canada, Philips, Vancouver, BC, Canada, 6Physics and Astronomy, University of British Colombia, Vancouver, BC, Canada, 7Pathology and Laboratory Medicine, University of British Colombia, Vancouver, BC, Canada
Synopsis
Current treatment and diagnosis of pain conditions are dependent on self-reported measures. The objective of this study was to establish the feasibility of determining changes in excitatory neurotransmitter concentrations (glutamate) in the anterior cingulate cortex (ACC) as an objective measure of pain using dynamic single voxel magnetic resonance spectroscopy (MRS). Glutamate levels can accurately be detected with this paradigm, although a general trend in relation to pain was not observed across subjects. This is the first study to report dynamic levels of glutamate in the ACC in relation to pain in healthy individuals using optimized MRS acquisition and processing methods.
Introduction
The International Association for the Study of Pain (IASP) defines pain as ‘‘an unpleasant sensory or emotional experience associated with actual or potential tissue damage1. This broad definition, demonstrates that pain is a subjective experience which should require a more comprehensive assessment than what a single domain measure can provide. Yet, the one-dimensional 0 to 10 numeric rating scale (NRS-11) represents the current clinical gold-standard for evaluating pain. This measure provides no mechanistic information leading to limitations in diagnosis and treatments for pain conditions. The excitatory neurotransmitter glutamate is one potential brain metabolite that plays an important role in pain-processing pathways2,3. Previous studies using magnetic resonance spectroscopy (MRS) in the anterior cingulate cortex (ACC) found increased glutamate in response to painful stimulation2,3 and an association between pooled glutamate/glutamine with pain sensitivity3, however, the later study measured pain sensitivity and glutamate levels on separate days, thus not allowing for causal inference. Simultaneous measurement of pain and glutamate would enable a more direct investigation of glutamate’s role in pain processing. Our goal was to determine the feasibility of dynamically measuring ACC glutamate levels in response to a painful intervention while participants remained in the MRI scanner.Methods
Study participants: 5 healthy participants (4F/1M, mean age=24, range:19-45yrs) with no known clinically diagnosed pain condition were studied.
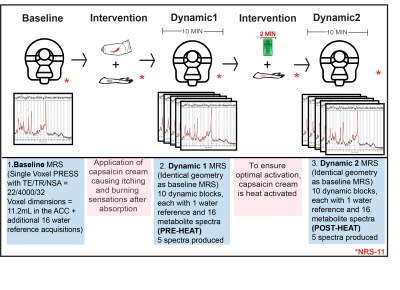
Pain intervention: Each participant received the painful intervention twice, the first time outside of the scanner, on a different day, to avoid novelty effects. The intervention involved the application of topical capsaicin-cream (0.0075%), the active component of chili peppers. After baseline MR data collection, the cream was placed on the inner part of the right forearm and left for 10-minutes, allowing absorption. During this period, a 10-minute dynamic MRS scan was collected (Dynamic1, PRE-HEAT). Then an activated heat-pack (48.7 ± 0.27°C) was placed on the inner forearm for 2 minutes, further activating the capsaicin-cream. This was followed by another 10-minute dynamic scan (Dynamic2, POST-HEAT). The participants remained in the bore of the magnet and provided pain ratings (NRS-11) intermittently (Figure1).
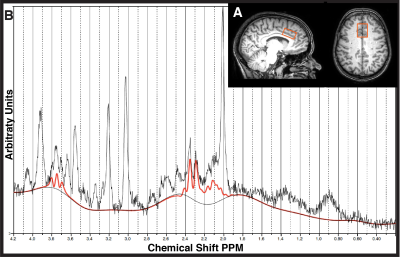
MR Experiments: 3T(Philips Achieva, Best, Netherlands) data was collected with a Transmit-Receive (T/R) head coil including: 3DT1 for voxel placement and tissue segmentation (MPRAGE, sequence 1mm³ isotropic resolution, TE/TR=3.5/7.7ms, shot interval=1800ms, inversion time=808ms, FOV(ap/rl/fh) =256/200/150mm³), 1H-MRS (PRESS, TE/TR/NSA=22/4000/32, ACC voxel dimensions= 30/25/15mm3 =11.2mL, second order shimming, 16-step phase cycle, Figure2A; non-water suppressed spectra also collected for monitoring water signal development and absolute quantification of the metabolites4,5,6,7,8), and T2-weighted images (TE/TR= 90/2000ms FOV(ap/rl/fh)=250/189/36 mm³, reconstructed voxel size= 0.98x0.97x3.00 mm3) were acquired immediately prior to each MRS scan to confirm voxel placement.
Data Analysis: Individual shots were saved and were pre-processed (eddy current correction, frequency alignment) prior to averaging 32 shots together for each 2-minute block, yielding a total of 11 spectra (1 baseline, 5 PRE-HEAT, 5 POST-HEAT). LCModel (v6.3) determined the contribution of glutamate in each 2-minute time block (Figure2B). A Pearson correlation examined the relationship between baseline glutamate and NRS-11 pain intensity scores during the acutely painful stimuli (heat-pack).
Results
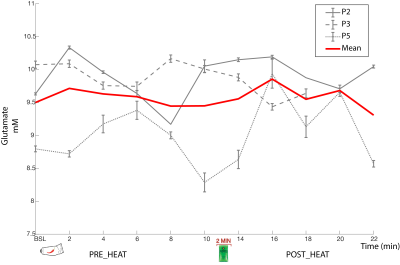
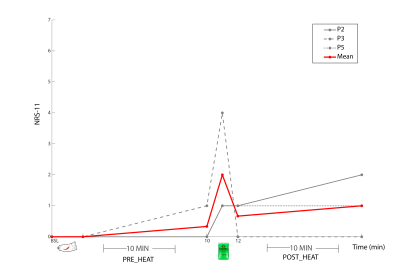
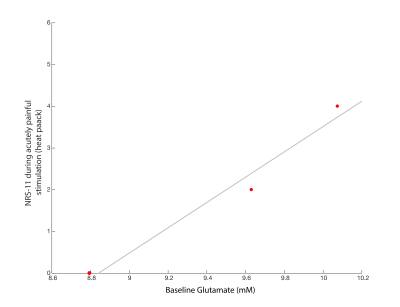
One subject was unable to complete the scan (claustrophobia) and one subject’s data was rejected due to poor quality (motion artifacts). Spectra from the remaining 3 participants had good signal to noise ratio (mean 17.71 (95% (CI 14.4-21.01)), low linewidth (mean 3.48Hz (CI 2.80-4.16)) and low mean error estimate on the glutamate fit (4%, (CI 3.99-4.00)) demonstrating glutamate could be detected by this experimental set-up. No overall trend of glutamate was observed over time in relation to the painful-intervention (Figure3). Individual subject fluctuations in pain scores (Figure4) and glutamate levels (Figure3) were present; subject P2 showed a 7.3% increase in glutamate immediately after capsaicin application, P5 had an 12.9% increase after heat application, while P3 showed no increases. The self-reported pain scores (NRS-11) increased with increasing baseline levels of glutamate (r=0.93, p=0.23, Figure5).Discussion and Conclusion
Dynamically measuring glutamate levels during an in-scanner painful intervention is feasible. We found an increase in self-report pain values during painful stimuli with increasing glutamate concentrations at baseline, in line with previous reports3. No general trend of changes in glutamate in relation to the painful intervention was seen, although variation in individual responses were observed. To conclude glutamate changes are an indicator of pain, glutamate activity must relate to the reporting of pain, and must not occur in the absence of pain. This knowledge should be incorporated into future studies when designing pain intervention experiments, to account for arousing non-painful stimulation activity. Increasing the sample size is warranted prior to drawing conclusions about measuring changes in glutamate levels during topical capsaicin plus heat pain intervention.Acknowledgements
We would like to thank the University of British Columbia MRI Research Centre, the International Collaboration for Repair Discoveries (ICORD) as well as volunteers who participated in the study. JA is supported by a Masters’ research scholarship of the National Council of Science and Technology (CONACYT). CG is supported by an endMS MSc studentship award from the MS Society of Canada. CL holds operating grant funding from the MS Society of Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC). This work is supported by NSERC Program Discovery grant held by JLKK.References
1. Merskey H, Bogduk N. Part III: Pain Terms, A Current List with Definitions and Note on Usage" (pp 209-214) Classification of Chronic Pain, Second Edition, IASP Task Force on Taxonomy. Retrieved from https://www.iasp-pain.org/Taxonomy.
2. Mullins PG, Rowland LM, Jung RE, Sibbitt WL. A novel technique to study the brain’s response to pain: Proton magnetic resonance spectroscopy. Neuroimage 26: 642–646, 2005
3. Zunhammer M, Schweizer L, Witte V, Harris RE, Bingel U, Schmidt-Wilcke T. Combined glutamate and glutamine levels in pain-processing brain regions are associated with individual pain sensitivity. Pain 157: 1, 2016.
4. MacMillan, E. L. Tam, R, Zhao, Y, Vavasour, I M, Li, Dkb, Oger, J, Freedman, M S, Kolind, S H, Traboulsee, A.L. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult. Scler. J. 22, 112–116, 2016.
5. De Graaf, RA. In Vivo NMR Spectroscopy: Principles and Techniques, 2nd Ed. John Wiley & Sons, Ltd. Chichester, UK. 2007.
6. Laule C, Vavasour IM, Kolind SH, Traboulsee AL, Moore GRW, Li DKB, MacKay AL. Long T2 water in multiple sclerosis: what else can we learn from multi-echo T2 relaxation? J Neurol. 254:1579–1587 2007.
7. Vavasour IM, Laule C, Kolind SH, et al. Hydration status does not affect brain water content or myelin water fraction in healthy volunteers. In: 19th Annual International Society for Magnetic Resonance in Medicine Annual Meeting & Exhibition. Honolulu, USA, April 18, 2009, paper no.3217. ISMRM.
8. Liang ALW, Vavasour IM, Mädler B, Traboulsee AL, Lang DJ, Li DKB, MacKay AL, Laule C. Short-term stability of T1 and T2 relaxation measures in multiple sclerosis normal appearing white matter. J Neurol. 259:1151–1158 2012.
Figures