1345
Aberrant Glutamatergic Neurotransmission in the Left Dorsolateral Prefrontal Cortex in Patients with Mild Cognitive Impairment: Preliminary Evidence from Task-Based Proton Magnetic Resonance Spectroscopy1Imaging Sciences and Interventional Radiology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, India, 2Neurology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, India
Synopsis
Much less is known about the changes in glutamate during working memory (WM) in patients with mild cognitive impairment (
INTRODUCTION
Neuroimaging and electrophysiological studies have consistently reported the involvement of Dorsolateral Prefrontal Cortex (DLPFC) in the processing of working memory (WM) 1, 2. WM deficits have been widely reported in patients with mild cognitive impairment (MCI) 3. Glutamate is a major excitatory neurotransmitter in the brain which plays an important role in learning and memory 4. Task-based proton magnetic resonance spectroscopy (1H-MRS) allows in vivo measurement of dynamic changes in metabolites critically important for brain function. Previous studies have reported changes in glutamate during the performance of cognitive as well as non-cognitive tasks 5-9. Till date, much less is known about the changes in glutamate during WM task in patients with MCI. In this study, we aimed to understand the glutamatergic response to functional activation in patients with MCI and healthy subjects (HS).METHODS
Five patients with MCI and eight age and education-matched HS participated in this study. Informed consent was obtained from all study participants. Magnetic Resonance Imaging and Spectroscopy studies were performed on a 3T GE MR750W scanner using a 24 channel coil. The anatomical T1 gradient weighted images were acquired using 3D Fast Spoiled Gradient Recalled Acquisition imaging. Single voxel 1H-MRS was performed using a point resolved spectroscopy sequence (TR/TE= 2000/30 ms) with a voxel size of 2×2×2 cm3 positioned in the left DLPFC as shown in figure 1. For metabolite quantification, LCModel with a simulated basis-set was used. Metabolite concentrations were corrected for the individual cerebrospinal fluid fraction within the 1H-MRS voxel by co-registering the individuals’ structural data with 1H-MRS data. Standard metabolites examined for this study were N-acetyl aspartate, myo-inositol, choline, creatine, glutamate, glutamine, and Glx (glutamate-glutamine). For the task-based 1H-MRS study, a letter version of N back WM task was administered. The experiment consisted of two tasks: the ‘0 back’ condition and the ‘1 back’ condition administered alternately in five blocks, implemented in Presentation software. The task displayed sequences of uppercase consonants with a stimulus duration of 500 ms and an interstimulus interval of 1500 ms. In the 0-back condition, participants responded to a target (i.e., letter X) and during the 1-back condition, participants responded, if the letter was identical to the one preceding it. Responses were collected by pressing the button using the right index finger using a color-coded keypad given to the participants inside the scanner. 1H-MRS was performed at three different time points, viz., before, during and after the performance of WM task. The duration of each spectroscopic sequence was 5 min 6 sec. Repeated measures analysis of variance (rmANOVA) was conducted to compare the changes in metabolites over time in the HS and patients with MCI.RESULTS
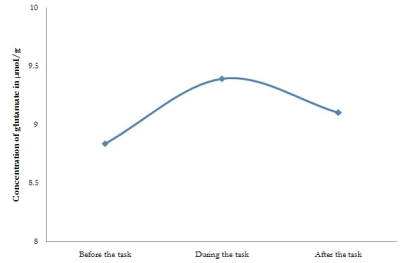
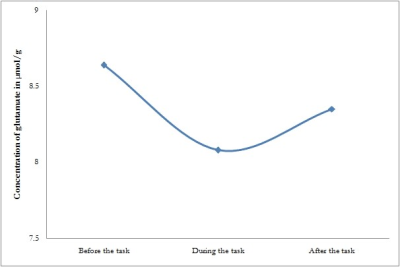
A repeated-measures analysis of variance revealed a main effect of time for glutamate concentrations for all groups (P<0.05) and not for other metabolites. In HS, glutamate raised from a resting level concentration of 8.83±1.2 µmol/g to 9.393±1.5 µmol/g during the WM task, which was marginally significant (P=0.05). Later, the glutamate concentration decreased to 9.107±1.09 µmol/g, which was not significant. But in patients with MCI, no significant changes were observed in glutamate at three different time points, viz., before the task (8.64±1.41), during the task (8.08±1.17), and after the task (8.35±0.85). Figure 2 and 3 depicts the changes in glutamate at three different time points in HS and patients with MCI respectively.DISCUSSION
This is the first study to examine the changes in glutamate during a letter N back WM task in patients with MCI and elderly HS. A previous study, 10 has reported that the patients with MCI displayed poorer performance with increasing WM load factor. Therefore, we designed a simple WM task with low load conditions (0 and 1 back) to maintain high accuracy rate. Recently, we reported an increase in glutamate during WM task and the reduction in glutamate upon cessation of stimuli in younger healthy controls 11 which were comparable with other task-based MR spectroscopic studies 5-9. In this study, the elderly HS have shown a marginally significant increase in glutamate during the performance of the task, which was absent in patients with MCI. These findings could be a reflection of disruption in the glutamatergic neurotransmission, which may be a part of the underlying pathophysiology in patients with MCI. In future, task-based 1H-MRS studies should further elucidate the glutamatergic response to functional activation in a larger sample of patients with MCI.CONCLUSION
Our results suggest working memory functional deficits in the left DLPFC in patients with MCI. Task-based 1H-MRS could be considered as an effective imaging tool to identify brain activation in cognitive disorders.Acknowledgements
This work was supported by Science and Engineering Research Board (SERB), Department of Science and Technology (DST), India (Grant No. PDF/2016/000494 dated 28-Nov-2016 to Dr. Anupa A Vijayakumari).
References
1. D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res 1998; 7:1-13.
2. Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 1996; 16:5154-5167.
3. Saunders NL, Summers MJ. Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol 2010; 32:350-357.
4. Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res 2003; 140:1-47.
5. Kuhn S, Schubert F, Mekle R, Wenger E, Ittermann B, Lindenberger U, et al. Neurotransmitter changes during interference task in anterior cingulate cortex: evidence from fMRI-guided functional MRS at 3 T. Brain Struct Funct 2016; 221:2541-2551.
6. Taylor R, Schaefer B, Densmore M, Neufeld RW, Rajakumar N, Williamson PC, et al. Increased glutamate levels observed upon functional activation in the anterior cingulate cortex using the Stroop Task and functional spectroscopy. Neuroreport 2015; 26:107-112.
7. van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J 2010; 39:527-540.
8. Schaller B, Mekle R, Xin L, Kunz N, Gruetter R. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J Neurosci Res 2013; 91:1076-1083.
9. Taylor R, Neufeld RW, Schaefer B, Densmore M, Rajakumar N, Osuch EA, et al. Functional magnetic resonance spectroscopy of glutamate in schizophrenia and major depressive disorder: anterior cingulate activity during a color-word Stroop task. NPJ Schizophr 2015; 1:15028.
10. Niu HJ, Li X, Chen YJ, Ma C, Zhang JY, Zhang ZJ. Reduced frontal activation during a working memory task in mild cognitive impairment: a non-invasive near-infrared spectroscopy study. CNS Neurosci Ther 2013; 19:125-131.
11. Vijayakumari AA, Thomas B, Menon RN, Kesavadas C. Task based metabolic changes in the Left Dorsolateral Prefrontal Region during letter N back Working Memory Task using Proton Magnetic Resonance Spectroscopy. NeuroReport (In Press)
Figures
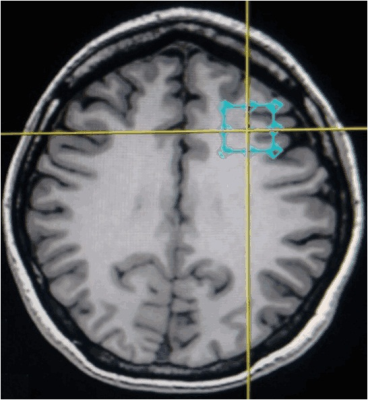
The voxel placement in the left dorsolateral prefrontal cortex (Axial view).The voxel is placed according to the radiological convention