Miho C Emoto1, Hirotada G Fujii1, and Hideo Sato-Akaba2
1Sapporo Medical University, Sapporo, Japan, 2Osaka University, Toyonaka, Japan
Synopsis
Glutathione (GSH) is an
important antioxidant that can protect cells under oxidative stress. Thus, a
non-invasive method to measure GSH levels in live animals is needed. To map the
levels of GSH in mouse brains, a new method using electron paramagnetic
resonance (EPR) imaging with nitroxide imaging probes was developed. By analyzing
the relationship between reduction rates for nitroxides in brains measured by
EPR and brain GSH levels measured by biochemical assay, pixel-based mapping of
brain GSH levels was successfully obtained. The newly developed method was
applied to a kindling mouse model of epilepsy to clarify the role of GSH.
Purpose
Glutathione (GSH) is
an important antioxidant that can protect the cell against reactive oxygen
species under oxidative stress. Several studies have reported decreased levels of brain GSH in rodent
models of epilepsy and Alzheimer’s disease; thus, a non-invasive method to
measure GSH levels is needed for monitoring and evaluating its antioxidant
capacity in those models. Recently, in our electron paramagnetic resonance
(EPR) imaging studies on GSH-depleted mouse brains, we found that the reduction
rate constants of nitroxide imaging probes depend on the levels of GSH in mouse
brains1. Using this relationship, GSH levels in mouse brains can be
estimated and visualized from the pixel-based map of reduction rate constants
of nitroxides distributed in mouse brains. In the present study, using the
mouse model of GSH depletion with diethyl maleate (DEM), three-dimensional (3D)
mapping of GSH levels in mouse brains was examined non-invasively by EPR
imaging with the nitroxide 3-methoxycarbonyl-PROXYL (MCP). Furthermore, we
applied this method to a pentylenetetrazole (PTZ)-induced kindling model of
epilepsy and visualized GSH levels in kindled mouse brains.
Materials and Methods
Chemicals:
MCP
was obtained from NARD Chemicals, Ltd. (Osaka, Japan). DEM was obtained from
Wako Pure Chemicals (Osaka, Japan). PTZ was from Sigma-Aldrich (St. Louis, MO).
Animals: Male C57BL/6 mice aged 6 to 10 weeks with body
weights of 20–25 g were used. For DEM treatment, mice were given a single
injection of DEM (4 mmol/kg) intraperitoneally. To prepare kindled mice,
sub-convulsive doses (35 mg/kg) of PTZ were intraperitoneally injected once a
day. MRI measurements: MRI of mouse heads was acquired using
an MRmini scanner (MR Technology, Tsukuba, Japan) with a 0.5 T permanent
magnet. EPR imaging: All EPR images
were acquired using an in-house built 750 MHz CW-EPR imager. Using the rapid
magnetic field scan system, the fastest data acquisition time for 3D-EPR images
is about 9 s for 50 ms field scanning (6 mT field scan) and 181 projections. Brain GSH level: GSH levels were
measured using high-performance liquid chromatography (HPLC) with electrochemical
detection.Results and Discussion
After infusion of MCP to
control (n=8) and DEM-treated (n=8) mice through the tail vein, temporal EPR
images of mouse heads were measured every 9 s. Based on the pharmacokinetics of
the MCP reduction reaction, the pixel-based rate constant of its reduction
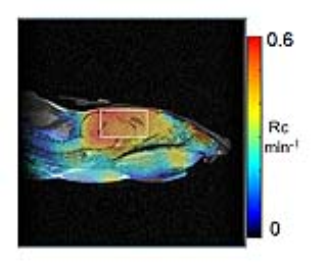
reaction was calculated and mapped as a redox map. The redox map of the
examined mouse head was co-registered to its MRI (Fig. 1). The average rate
constants within selected ROI in the brain were calculated for control and
GSH-depleted mice. After imaging experiments, brain GSH levels were measured in
vitro by HPLC. The reduction rate constant (Rc) for MCP in each mouse with or
without DEM treatment was plotted against brain GSH level ([GSH]). Rc in mouse
brain increased linearly with [GSH] for each mouse, and the following linear
relationship between Rc and [GSH] was obtained: Rc = 0.0021 × [GSH, mmol/g
tissue] + 0.1368, where R2 = 0.9812 for 16 mice. By converting the
value of Rc to [GSH] at each pixel of a redox map using this equation, a GSH
map of examined mouse brains was obtained from their redox maps for control and
DEM-treated mice (Fig. 2). In control mouse brains, GSH was distributed
throughout the brain, and more GSH was found in the cerebellum and hippocampus
than in the cerebrum. GSH distribution in these regions of mouse brains was
significantly reduced by DEM treatment. The distribution pattern of GSH
described in Fig. 2 was similar to that obtained by previously published
invasive methods such as auto-radiographic imaging and histochemical staining.
Next, we attempted to estimate the change in GSH levels in PTZ-induced kindled
mouse brains. A redox map of PTZ-induced kindled mouse brains was obtained by
EPR imaging with MCP, and this redox map was converted to a GSH map of kindled
mice. Figure 3 shows GSH maps of control and kindled mouse brains, and clearly
reveals a remarkable change in GSH levels around the hippocampal region. These
results also indicate that the hippocampus was susceptible to oxidative damage
with PTZ treatment, which was followed by the decrease in GSH levels.Conclusion
This study shows that the EPR imaging method with the nitroxide MCP can
visualize brain GSH levels in mice non-invasively. The brain GSH map in
PTZ-kindled mice obtained by EPR imaging clearly indicates the decreased level
of GSH in the hippocampal region. The
method of mapping GSH levels developed in this study will contribute to further
clarifying the role of GSH in neurodegenerative disease.Acknowledgements
This work was supported by a grant from the Japanese Society for the Promotion of Science (24791318).References
1 Proc. Intl. Soc. Mag. Reson. Med. 25 5594 (2017)