1340
Effect of sampling method on HR-MAS NMR spectra of caprine brain biopsies1Department of Clinical Veterinary Medicine, University of Bern, Bern, Switzerland, 2DBMR, University of Bern, Bern, Switzerland, 3DCR-VPH, University of Bern, Bern, Switzerland
Synopsis
Metabolic profiling of tissue biopsies using HR-MAS NMR has potential diagnostic and prognostic value, but alterations in the biochemical profile due to factors such as sampling method may lead to misinterpretation. Therefore we investigated the effect of two different sampling methods in normal caprine brain tissue, in vivo sampling by stereotactic biopsy and direct post mortem surgical sampling. We found significant differences between the two biopsy types with elevated lactate and creatine, and altered choline-containing compounds. We conclude that metabolite alterations depend on sampling methods and suggest the use of in vivo biopsy in animal models.
Introduction
Metabolic profiling of tissue biopsies using high resolution-magic angle spinning (HR-MAS) 1H NMR has potential diagnostic and prognostic value and may aid in the interpretation of in vivo Magnetic Resonance Spectroscopy (MRS). It has been shown that the biochemical profile of brain biopsies may be affected by experimental factors such as delayed sample freezing and prolonged measurement time1,2. Metabolite alterations have been attributed to sample ischemia and mechanical stress before and during the NMR experiment in neoplastic and normal brain biopsies obtained by surgical bulk resection or post mortem biopsy1,2. However, this did not include samples obtained by in vivo stereotactic biopsy method, which is as close as possible to the in vivo status. Therefore, we investigated metabolite alterations between brain biopsies obtained in vivo by a minimally invasive stereotactic approach and by post mortem biopsy in healthy goat brain.Methods
Bilateral brainstem and thalamus biopsies were obtained from four healthy goats by minimally invasive stereotactic brain biopsy under general anesthesia (in vivo biopsies) and by surgical resection of adjacent areas within 20 minutes of euthanasia (post mortem biopsies) (total of 8 samples per location and sampling method). Both biopsy types were immediately snap-frozen in liquid nitrogen and stored at -80°C, before being placed in a 12 μl MAS rotor with D2O-based phosphate-buffered saline. 1H HR-MAS NMR experiments were performed on a Bruker Avance II spectrometer (500.13 MHz) at 3 kHz MAS and 284 K. A 1D 1H sequence with water presaturation and with a T2-filter eliminating J-modulation (“project”3) was applied. After postprocessing of spectra using TopSpin (Bruker Biospin GmbH), chemometric analysis to determine differences between the two biopsy types was performed using a home-written Matlab script (R2011b, The MathsWorks Inc.) and the PLS toolbox of Matlab. For multivariate analysis using Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA), 250 buckets of variable size according to peak width were defined after exclusion of areas of pure noise, lipids and the contaminant ethanol. PLS-DA models were subsequently subject to permutation testing (cross validation, 50 iterations). For interpretation of loading plots, buckets with loading values beyond an arbitrary threshold of +/-0.1 were considered as strong contributors. After assignments of resonances, the multiple resonances of a specific metabolite were analyzed and were only assumed to be discriminating if all resonances showed a consistent pattern. Experiments were performed in agreement with the local ethics regulations.Results
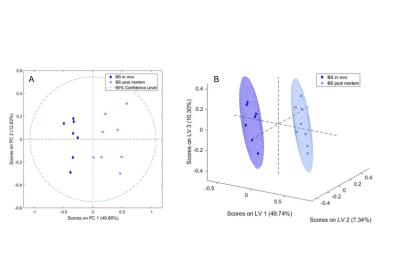
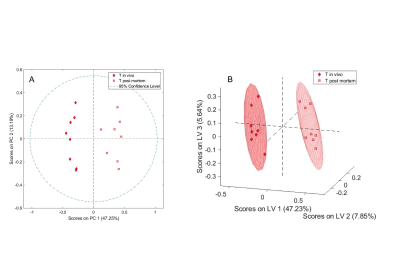
One in vivo biopsy sample of the brainstem was excluded due to poor spectral quality. A significant separation between in vivo and post mortem biopsies of the brainstem (Fig. 1) and the thalamus (Fig. 2) was achieved both in unsupervised PCA (t-test, Brainstem p<0.001, Thalamus p<0.001) and supervised PLS-DA (Wilcoxon, Brainstem p=0.001, Thalamus p=0.004). In both the brainstem and thalamus biopsies, choline was decreased and phosphocholine, glycerophosphocholine, creatine and lactate were increased in the post mortem compared to the in vivo biopsies (Fig. 3). In addition, in the brainstem myo-inositol, scyllo-inositol and acetate and in the thalamus gamma-aminobutyric acid were increased in the post mortem compared to the in vivo biopsies (Fig. 3).Discussion
The metabolite alterations observed in the post mortem compared to the in vivo brain biopsies demonstrate rapid changes most likely due to sample ischemia after death and during sample excision prior to snap freezing. Lactate is present in both sample types and increased in the post mortem samples consistent with anaerobic glycolysis due to ischemia of the sample. Alterations in the choline group likely reflect membrane damage. In contrast to a study of post mortem metabolite levels in rabbit brain4, we also observed an increase in creatine in the post mortem biopsies compared to the in vivo biopsies, as reported in rat brain1. This suggests a release of NMR-invisible bound creatine as a result of tissue damage in the post mortem biopsies. However, it seems questionable that the mechanical stress during the MAS experiment is the main cause of the creatine increase, as stated previously1 since the NMR part was identical for the in vivo and post mortem biopsies with the sampling method and delay in initial sample freezing being the only differences.Conclusion
Alterations of a number of metabolites, most prominently the choline-group, creatine and lactate occur rapidly due to sample ischemia and have to be accounted for when results of different sampling methods are being interpreted. We suggest the use of in vivo over post mortem biopsy in animal models to be more close to the in vivo status and aid in the interpretation of in vivo MRS data of human patients. The increase in creatine in the post mortem biopsies underlines it´s variability and questionable value as an internal reference standard.Acknowledgements
No acknowledgement found.References
1. K. S. Opstad. B. A. Bell, J. R. Griffiths and F. A. Howe, NMR Biomed., 2008, 21, 1138-1147.
2. D. Valverde-Saubi, A. P. Candiota, M. A. Molins, M. Feliz, O. Godino, M. Davila, J. J. Acebes and C. Arus, Magn. Reson. Mater. Phy., 2010, 23, 203-215.
3. J. A. Aguilar, M. Nilsson, G. Bodenhausen and G. A. Morris, Chem. Commun., 2012, 48, 811-813.
4. O. A. Petroff, T. Ogino, J.R. Alger, J. Neurochem., 1988, 51, 163-171.
Figures