1321
Implications of magnetic susceptibility difference between grey and white matter for spectroscopy quantification at 7T.1Erwin L. Hahn Institute for MRI, University of Duisburg-Essen, Essen, Germany, 2Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Nijmegen, Netherlands, 3Donders Institute for Brain, Cognition and Behavior, Radboud University, Nijmegen, Netherlands
Synopsis
Magnetic susceptibility differences between grey matter (GM) and white matter (WM) can potentially affect lineshapes and chemical shifts in single voxel spectroscopy. Hitherto, analytical techniques such as LCModel assumed a single lineshape per voxel. Separated GM and WM signals using multi-echo GRE image sequence in combination with literature values for the metabolite distribution between GM and WM enable to construct a realistic basis set for LCModel. With this information we can test how magnetic susceptibility induced lineshape modification affects metabolic quantification, which uses spectral prior knowledge.
Introduction
Magnetic susceptibility differences between grey matter (GM) and white matter (WM) can potentially affect lineshapes and chemical shifts in single voxel spectroscopy. This can broaden the linewidth of metabolites, and potentially lead to a line shape dependent on the relative GM/WM distribution. Hitherto, analytical techniques such as LCModel1 assumed a single lineshape per voxel. SVS in combination with multi-echo GRE image sequence possible to separate GM and WM. We can now use the additional information on GM/WM frequency shift combined with literature values for the metabolite distribution between GM and WM to construct a realistic basis set for LCModel. With this information we can test how magnetic susceptibility induced lineshape modification affects metabolic quantification, which uses spectral prior knowledge.Material and Methods
A single voxel spectroscopic signal can be considered as an aggregation of signals from multiple mini voxels inside the measurement volume. Signal offset of each single mini voxel, which is defined by imaging resolution, could be estimated by the phase evolution of multi-echo GRE images. By sorting mini voxels into corresponding GM and WM components, we can separate the GM and WM signals. Then, we can measure the magnetic susceptibility induced frequency shift between GM and WM in single voxel spectroscopy. GMWM coordination was mapped by the T1 weighted image segmentation.
We acquired MRI and MRS data from parietal cortex of 5 healthy volunteers (1M/4F; 29.7 ± 3.78YO) using the Semi-LASER2 and a 7T system (Siemens, Erlangen) with following parameters: 8cm3 isotropic voxel, TE/TR/NEX=38ms/4500ms/64. A 3D MPRAGE was acquired as an anatomical reference before spectroscopy. B0 shimming was performed by FASTESTMAP3. A 3D GRE phase imaging acquisition was performed after the spectroscopy scan to have a frequency offset map at the same location as the spectroscopic voxel with; 1mm3 isotropic voxel with no spacing, FOV = 256 * 256 *20mm3, TEs = 10/14.24/18.48/22.72ms, FA=20°.
Realistic lineshapes were obtained by
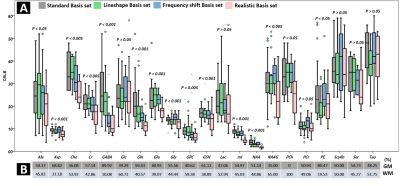
convolving mixed lineshapes, weighted by the literature value for relative GM
and WM concentration ratio4,5 (Fig 1B), and volume fraction in the
voxel, with the basis functions obtained from spectral simulation by NMRSIM
(Bruker, Rheinstetten). We also included the standard basis set (Conventional
basis set), the realistic lineshape basis set (lineshape changes only), and the
frequency shift basis set (frequency shift only) to examine the effects of the
lineshape changes and the frequency shifts separately. Therefore, we compared
results by four different basis sets. Spectral quantification was done by
LCModel (ver. 6.3, Provencher, Ontario). Goodness of fit was compared by Cramer
Rao Lower Bound (CRLB), which is an estimated spectral fitting error.
Results
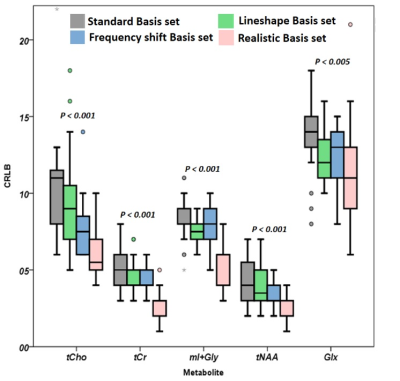
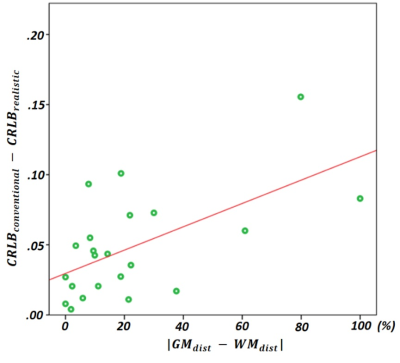
We found both frequency shift (4.81±1.98Hz) and linewidth difference (2.37±1.46Hz) between GM and WM signals. Fig1A shows a comparison of CRLB by four different basis sets with p-value. Fig2 also shows comparisons of CRLB for the composite signals. The mean CRLB values, which were obtained by the realistic basis set were lower than those obtained with the standard basis set for the composite signals. We could find that our approach achieved a statistically meaningful improvement for all these signals (p < 0.005 for all). Figure 3 shows the relationship between the improvement in CRLB and the absolute metabolic distribution difference in GM and WM.Discussion
This study investigated the influence of a realistic basis set, which reflects the magnetic susceptibility difference between two different tissue types on the spectral quantification We found a similar level of the frequency shift to the level other imaging studies reported. The realistic basis set corrects for small chemical shift differences induced by the magnetic susceptibility difference. Matched peak positions resulted in decreased spectral fitting error. In light of the results, it is clear that no one specific factor was dominant. Instead, both the realistic frequency shift and the realistic lineshape had some effect. In the spectral fitting procedure, all metabolite concentrations are simultaneously estimated. Hence, small errors in the frequency assignment in the basis set for one metabolite can affect the estimation of other metabolite concentrations. The reduction in CRLB values indicates increased reliability in the estimation of metabolic concentrations. The effect of magnetic susceptibility difference between GM and WM should be taken into account, especially at ultrahigh field strengthAcknowledgements
This work was funded by the Helmholtz Alliance ICEMED – Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Networking Fund of the Helmholtz Association.References
1. Provencher, Stephen W. "Estimation of metabolite concentrations from localized in vivo proton NMR spectra." Magnetic resonance in medicine 30.6 (1993): 672-679.
2. Scheenen, Tom WJ, et al. "Short echo time 1H‐MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses." Magnetic resonance in medicine 59.1 (2008): 1-6.
3. Gruetter, Rolf, and Ivan Tkáč. "Field mapping without reference scan using asymmetric echo‐planar techniques." Magnetic resonance in medicine 43.2 (2000): 319-323.
4. Perry, T. L., et al. "Free amino acids and related compounds in biopsies of human brain." Journal of neurochemistry 18.3 (1971): 521-528.
5. Kreis, R. "Quantitative localized 1 H MR spectroscopy for clinical use." Progress in nuclear magnetic resonance spectroscopy 31.2 (1997): 155-195.
Figures