1306
MOSAIC - a generalized multi-channel coil combination for 1H-MRSI via interleaved calibration scans1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 4Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
The optimal combination of signals from all receive elements is a prerequisite in MRSI especially at high field (≥7T), not only for SNR-efficient acquisition, but also for good parallel imaging reconstruction [1,2]. Phantom and in vivo experiments showed superior performance of MOSAIC including higher SNR, smaller FWHM and anatomically detailed metabolic maps compared to Brown and WSDV coil combination. MOSAIC is a flexible and robust approach for efficient MRSI coil combination under challenging conditions (B0≥7T, many coil elements, no reference coil, low SNR, possible spectral artifacts, motion/instability related artifacts, 1st-order phase error), especially with an outlook on parallel-imaging non-Cartesian MRSI.
Introduction
The optimal combination of signals from all receive coil elements is a prerequisite in MRSI especially at high field (≥7T), not only for SNR-efficient acquisition, but also for good parallel imaging reconstruction [1,2]. Many coil combination methods have been proposed: 1) Sensitivity maps (Sensmap) based approaches are the gold standard, but require a reference coil that is not available at modern pTx systems [3], 2) others need either a non-suppressed water scan or weak-water suppression, which prolongs total scan times or degrades spectral quality [4,5], 3) others perform poorly for low SNR or in the presence of spectral artifacts. The recently published MUSCIAL coil combination [6] requires no reference coil, but is designed for phase-encoded MRSI. With the advent of spatial-spectral encoding methods for acceleration of ≥7T MRSI, the performance of MUSICAL has to be revisited. The purpose of our study was to develop a new coil combination method that capitalizes on all the benefits of MUSICAL, but is more general with respect to arbitrary spatial-spectral encoded MRSI trajectories. Our new approach termed Multi-channel data Of Spectroscopic imaging Assembled via Interleaved Calibrations (MOSAIC) acquires reference data in an interleaved fashion and is, thus, insensitive to motion and scanner instabilities.Methods
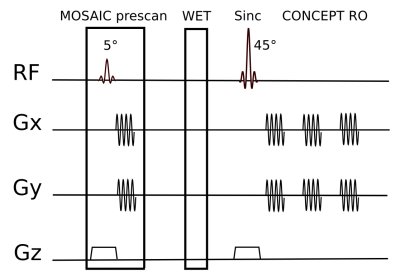
MOSAIC coil combination was integrated into a single-slice FID-MRSI sequence with non-Cartesian spatial-spectral encoding using equidistant concentric ring trajectories [7,8]. The calibration data was directly measured before the WET water suppression module of the MRSI sequence within each TR. The calibration module is identical to the MRSI excitation/acquisition module, but requires only a few trajectory repetitions and negligibly low flip angles of 5° to avoid possible saturation effects (Fig.1). The following FID-MRSI parameters were used: TR: 600ms; acquisition delay: 1.3ms; FA: 45°; FoV: 220×220mm²; matrix size: 64×64; spectral bandwidth: 2778Hz; three temporal interleaves; TA: 6:18min. The performance of MOSIAC coil combination was evaluated on a 7T Siemens Magnetom scanner with a 32-channel receive coil array. Three different reference-coil independent approaches were compared: MOSAIC, Brown (1st FID point) [5] and WSVD [9]. Phantom measurements were performed with a Siemens phantom containing 8.2g/l sodium acetat and 9.6g/l lithium pyruvate. In vivo data were acquired from five healthy volunteers. Coil combination efficiencies were evaluated qualitatively and quantitatively based on SNR (adapted pseudo-replica method [10]) and line width (FWHM) maps of NAA obtained via LCModel. CRLB values of tNAA, tCr, tCho and Glx were compared among the three methods. Mean and standard deviation over all voxels within the brain were derived per subject and compared using paired t-tests. The number of voxels where spectral quality was too low to allow reliable spectral fitting (i.e., CRLB NAA < 20) was assessed in all cases.Results
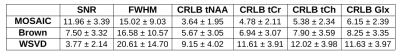
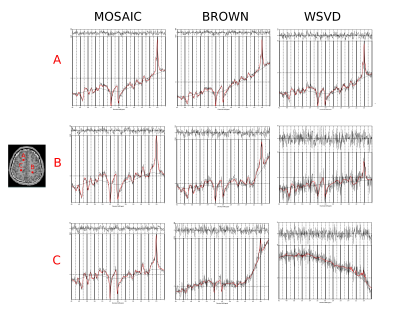
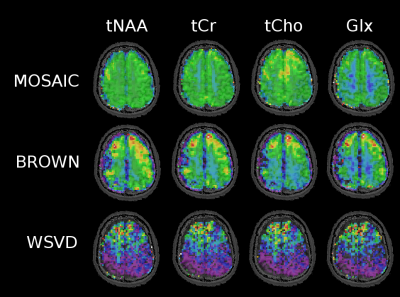
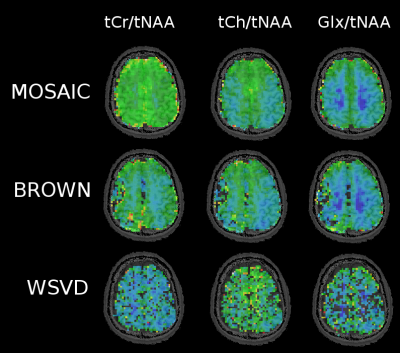
In phantom measurements all three methods performed similarly well yielding an average SNR of 18.9±3.7 for MOSAIC, 16.6±3.6 for Brown's method and 17.2±2.9 for WSVD. However, in vivo MOSAIC performed better than Brown combination, followed by WSVD, which performed worst. While MOSAIC and Brown yielded comparable amounts of accepted voxels, the mean SNR over all volunteers dropped by 63% and FWHM increased by 10% for Brown compared to MOSAIC (p<0.001). The mean CRLB values were 40% lower when using MOSAIC compared to Brown combination (Tab.1). Also, from a qualitative point of view, MOSAIC yields far more homogeneous and anatomically detailed metabolic maps (Fig.2,3). Metabolic maps obtained with WSVD were very inhomogeneous without any metabolic contrast. More than 15% less voxels could be reliably fitted, with an overall SNR loss of 68% and a 2.4fold increase in average metabolic CRLB values compared to MOSAIC (p<0.001).Discussion/Outlook
In vivo MOSAIC performed significantly better than the other two reference-coil independent methods (i.e. Brown and WSVD combination). The better performance of MOSAIC can be explained by the ability to obtain its reference data from an external water-unsuppressed source (=extrinsic), while both other approaches rely on information from the individual uncombined spectra itself (=intrinsic), which need water suppression. Both, Brown and WSVD seem to have scaling problems (see metabolic maps Fig.3), which is not so critical when looking at ratio maps (Fig.4). Also, Brown and WSVD combination seem to be more prone to lipid and angular interleaving artifact, as well as 1-st order errors than MOSAIC. In contrast to MUSICAL, MOSAIC can be directly applied with arbitrary MRSI trajectories due to its interleaved acquisition. Overall, MOSAIC is a flexible and robust approach for efficient coil combination of MRSI data under challenging conditions (i.e., B0≥7T, many coil elements, no reference coil, low SNR, possible spectral artifacts, motion/instability related artifacts, 1st-order error), especially with an outlook on parallel-imaging non-Cartesian MRSI.Acknowledgements
This study was supported by the FFG Bridge Early Stage Grant No. 846505, FWF Grant KLI 646 and FWF Grant P 30701.References
[1] Kirchner T., et al., 2015, Reduction of voxel bleeding in highly accelerated parallel 1H MRSI by direct control of the spatial response function, Magn. Reson. Med. 73, 469–80
[2] Strasser B., et al., 2017, (2 + 1)D-CAIPIRINHA accelerated MR spectroscopic imaging of the brain at 7T, Magn. Reson. Med. 78(2):429-440
[3] Pruessmann KP., et al., 1999, SENSE: sensitivity encoding for fast MRI, Magn. Reson. Med. 42: 952–962
[4] Graham GD., et al., 2001, Spectroscopic Assessment of Alterations in Macromolecule and Small-Molecule Metabolites in Human Brain After Stroke, Stroke; 32:2797–2802
[5] Brown MA., 2004, Time-domain combination of MR spectroscopy data acquired using phased-array coils, Magn. Reson. Med. 52: 1207–1213
[6] Strasser B., et al., 2013, Coil combination of multichannel MRSI data at 7 T: MUSICAL, NMR Biomed. 26, 1796–805
[7] Bogner W., et al., 2012, High-resolution mapping of human brain metabolites by free induction decay 1H MRSI at 7T, NMR Biomed. 25, 873–82
[8] Hingerl L., et al., 2017, Density-Weighted Concentric Circle Trajectories for High Resolution Brain Magnetic Resonance Spectroscopic Imaging at 7T, Magn. Reson. Med.
[9] Rodgers CT., et al., 2016, Coil combination for receive array spectroscopy: Are data-driven methods superior to methods using computed field maps?, Magn. Reson. Med. 75(2):473-87
[10] Robson PM., et al., 2008, Comprehensive Quantification of Signal-to-Noise Ratio and g-Factor for Image-Based and k-Space-Based Parallel Imaging Reconstructions, Magn. Reson. Med. 60(4):895-907
Figures