1303
1H-localised 13C DEPT measurement of glutamate and glutamine turnover in human frontal lobe using [1-13C]glucose infusion at 7T1Sir Peter Mansfield Imaging Centre, The University of Nottingham, Nottingham, United Kingdom, 2School of Life Sciences, University of Nottingham Medical School, Queen's Medical Centre, Nottingham, United Kingdom, 3Neuroscience and Psychiatry Unit, Division of Neuroscience and Experimental Psychology, University of Manchester, Manchester, United Kingdom, 4Institute of Mental Health, The University of Nottingham, Nottingham, United Kingdom, 5Centre for Imaging Science, University of Manchester, Manchester, United Kingdom
Synopsis
Human 13C MRS has recently shown its further potential in understanding neurological disorders. In the field of schizophrenia, 1H MRS has been applied with findings of abnormal concentrations of glutamate ﴾Glu﴿ and glutamine ﴾Gln﴿ in anterior cingulate cortex ﴾ACC﴿. It is therefore of interest to measure glutamate metabolism with 13C MRS in this brain region to get deeper understanding of these changes. In the present study, we applied localized 13C MRS at 7T upon [1-13C]glucose infusion, using a 13C/1H volume coil and polarisation transfer (DEPT) to test the feasibility of measuring glutamate turnover in ACC.
Introduction:
In vivo 13C MRS with infusion of 13C-labeled energy substrates has proven to be an useful tool that has provided new insights into the dynamics of brain energy metabolism, in preclinical rodent studies as well as human studies [1,2]. Human 13C MRS has recently shown its further potential in understanding neurological disorders [3]. In the field of schizophrenia, 1H MRS has been applied with findings of abnormal concentrations of glutamate ﴾Glu﴿ and glutamine ﴾Gln﴿ in anterior cingulate cortex ﴾ACC﴿[4]. It is therefore of interest to measure glutamate metabolism with 13C MRS in this brain region to get deeper understanding of these changes. However, the ACC is a deep structure in the frontal lobe, difficult to measure with 13C MRS and standard surface coil approaches in human subjects. In the present study, we applied localized 13C MRS at 7T upon [1-13C]glucose infusion, using a 13C/1H volume coil and polarisation transfer (DEPT)[5] to test the feasibility of measuring glutamate turnover in ACC.Methods:
The modified ISIS-DEPT sequence used adiabatic inversion pulses for 1H ISIS localisation to reduce chemical shift displacement artefacts, and block pulses for polarization transfer (Fig.1A). An OVS slab was positioned on the scalp region closest to the voxel to further reduce possible lipid signal contamination (Fig.1B). A 16-step phase cycling scheme (ISIS: 8 steps×DEPT: 2 steps) was implemented and spoiler gradients were added to further eliminate unwanted coherences[4]. 1H decoupling was not applied in this study.
Eleven healthy volunteers (8M; 30±8 years old) were scanned on a 7T Philips Achieva MR system (Philips Healthcare, Best, Netherlands) using a 13C/1H volume coil (RAPID Biomedical, Würzburg, Germany). After an overnight fast, participants were cannulated into a dorsal vein of the foot, placed in a warm box (50-55°C) and from which arterialized blood samples were taken at 5-min intervals throughout the dynamic 13C scan to adjust the rate of supply of [1-13C]glucose (20% mass/vol, 99% enrichment) that was infused through an antecubital vein on the forearm for 60 minutes. The DEPT sequence parameters were optimized for the detection of glutamate and glutamine CH2 groups (C4,C3), i.e. with the last 1H pulse θ=45°, interpulse delay 3.85ms, 13C offset centred at 31ppm and 1H offset at 2.3ppm, TR=2.5s. Raw spectra were averaged over 240 scans to obtain dynamic 13C spectra with 10min temporal resolution. The 8.5x3x5cm3 voxel was positioned over the ACC and vicinity (Fig.1B). Prior to the 13C-Glc infusion, flip angles were calibrated on the water signal in the corresponding GluC4 voxel (1H channel) and on a reference formic acid bubble placed in the coil (13C channel).
The 13C MRS data were quantified with the AMARES[6] algorithm (jMRUI v5.2). Absolute quantification was performed using an external reference method (glutamate phantom) [7], with the formic acid signal used as correction for effects of coil loading. Offset-dependent RF efficiency correction was applied based on a profile determined on a glutamate phantom (5mM 13C) in similar configurations. Preliminary interpretation of the group-averaged 13C turnover curves was undertaken using a one-compartment model of TCA cycle metabolism and Glu/Gln cycling (Fig.4), fitted to the GluC4 and GlnC4 curves, with a typical blood [1-13C]glucose enrichment input curve measured in a pilot experiment.
Results & Discussion:
A typical 13C MRS dynamic series acquired in vivo is shown in Fig.2. The triplet patterns of the C4 and C3 resonances of glutamate and glutamine consistently appeared after about 15 minutes, with low contamination of lipid signal, as also confirmed by a baseline scan. The quantification results in the same dynamic series are displayed in Fig.3, with GluC4 having the fastest turnover followed by GlnC4, while the C3 of glutamate and glutamine appeared above the noise level at a later stage, consistent with the delayed metabolic labelling of these positions. The group-averaged C4 turnover curves are shown in Fig.4 with the best fit of the one-compartment model, leading to the following metabolic parameters: Vgt=0.44±0.06 [µmol/g/min], VNT=0.24±0.03 [µmol/g/min] and dilN=51±3%.Conclusion:
The present study shows the feasibility of 1H-localized polarization transfer 13C MRS dynamic acquisitions from the ACC region in the frontal lobe of the human brain. It was possible to overcome the technical challenges encountered in this deep region including the increased SAR deposition at 7T in particular, the larger 13C chemical shift range and limited RF power required a careful pulse sequence calibration, which provided a reproducible measurement of the C4 groups of glutamate and glutamine. This work opens the way to measurements of altered glutamate metabolism in ACC, of particular interest in research on schizophrenia, the next step of our project.Acknowledgements
This work is part of SPRING (The Study of Psychosis and the Role of Inflammation and GABA/Glutamate) project, funded by UK Medical Research Council (MRC). C. Fernandes is funded by the Initial Training Network, HiMR, financed by the FP7 Marie Curie Actions of the European Commission (FP7-PEOPLE-2012-ITN-316716).References
1) Rothman DL, De Feyter HM, De Graaf RA et al., 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans, NMR Biomed. 2011; 24: 943–957;
2) Lanz
B, Gruetter R and Duarte JMN, Metabolic Flux and Compartmentation Analysis in the Brain In vivo, Front. Endocrinol. 2013; 4: 156;
3) Abdallah CG, Jiang L, De Feyter HM et al.,
Glutamate metabolism in major depressive disorder., Am. J. Psychiatry. 2014; 171:1320-1327;
4) Marsman A, van den Heuvel MP, Klomp DWJ et al., Glutamate in Schizophrenia: A Focused Review and Meta-Analysis of 1H-MRS Studies, Schizophr.
Bull. 2013;v39﴾1﴿:120–129;
5) Henry PG, Tkac I and Gruetter R, 1H-localized broadband 13C NMR spectroscopy of the rat brain in vivo at 9.4 T, Magn. Reson. Med. 2003; 50: 684–692;
6) Vanhamme L, van den Boogaart A and Van Huffel S, Improved method for accurate and efficient quantification of MRS data with use of prior knowledge, J. Magn. Reson.
1997; 129(1): 35–43;
7)
Gruetter R, Adriany G, Choi IY et al., Localized in vivo 13C NMR spectroscopy of the brain, NMR Biomed. 2003;
16: 313–338;
Figures
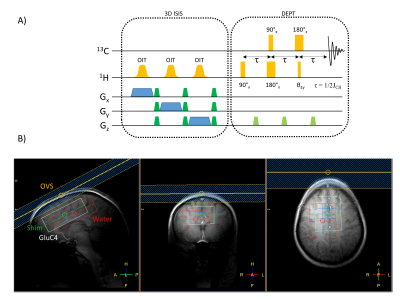
Figure1
A) Schematic of the ISIS-DEPT sequence used for localised in vivo acquisition of 13C labelling in the human brain.
B) Example of ISIS-DEPT voxel positioning in the frontal lobe, centred on the ACC. The white box shows the GluC4 voxel shifted from the red water box (CSDA from the 1H ISIS localisation). The shim box (green) was centred on the GluC4 voxel and 1D outer-volume saturation was applied to further suppress possible lipid signal contamination from the scalp.
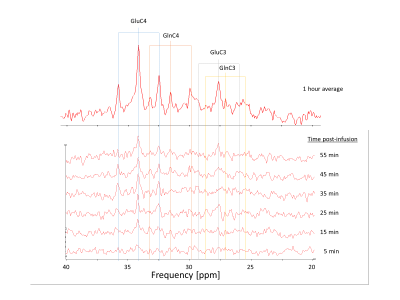
Figure2
Example of dynamic 13C MRS results on a single subject under infusion of [1-13C]glucose, reconstructed with a temporal resolution of 10min over 60min. 13C offset was centred at 31ppm. Typical triplet resonances of the CH2 groups of glutamate/glutamine appear 15min post-infusion.
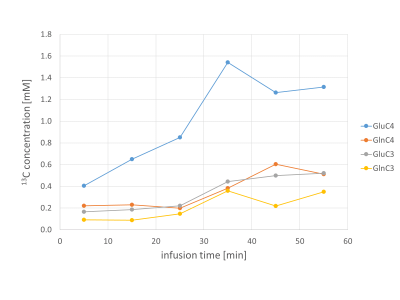
Figure3
Quantified 13C concentration curves from the same single participant as in figure 2.
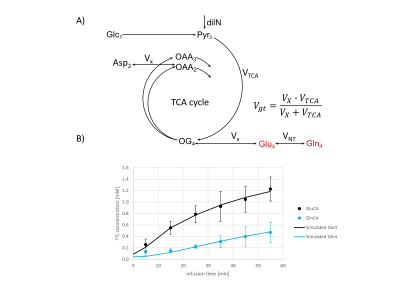
Figure4
A) Schematic of the one-compartmental model of brain energy metabolism used for the analysis of the dynamic 13C data. The free parameters are the glutamate turnover flux Vgt, the glutamate/glutamine exchange VNT and the dilution factor dilN, taking into account the metabolism of unlabelled substrates. Pool sizes were set to 0.1mM for TCA intermediates and 4mM and 8mM for Gln and Glu, respectively. The compounds displayed in red were used for the model fitting.
B) Group-averaged timecourses of the GluC4 and GlnC4 13C concentrations (n=11, mean ± sd) and simulated uptake curves obtained from the best fit of the metabolic model shown in A).