1302
Feasibility of Echo Time Optimization for Glutamate and Myoinositol Detection using TE-Averaged PRESS Spectral Editing Technique in Human Brain at 3T.1Biomedical Engineering Institute, Bogazici University, Istanbul, Turkey
Synopsis
This study aims to investigate the feasibility of echo time (TE) optimization for TE-averaged PRESS for faster detection of glutamate (Glu) and myoinositol (mI) in human brain at 3T. Proton MR spectroscopic imaging (1H-MRSI) data of a brain phantom and a healthy volunteer were acquired at 3T using 10 different TEs, which were selected based on prior Monte Carlo simulation results. TE-averaged PRESS spectra were created with best TE combinations, and metabolites were quantified in MATLAB. Our results indicated that TE-averaged PRESS with upto 5 TE’s could reliably detect separate Glu and mI metabolites.
Purpose
Proton MR spectroscopic imaging (1H-MRSI), which provides a noninvasive investigation of brain metabolism, has an important role for diagnosis and follow-up of several brain diseases. However, 1H-MRSI often has long scan times and it is difficult to separate overlapping metabolites. Spectral editing techniques are used to separately image and quantify these overlapping metabolite peaks. TE-averaged point-resolved spectroscopy (PRESS) 1 has been proposed for unobstructed detection of glutamate (Glu) and myo-Inositol (mI) peaks. On the other hand, TE-averaged PRESS technique has long acquisition time, due to the acquisition of several TE’s for spectral averaging. In this study, we investigate the feasibility of TE optimization for faster glutamate (Glu) and myoinositol (mI) detection using TE-Averaged PRESS spectral editing technique in human brain at 3T.Methods
A simulated spectra including brain metabolites was created based on the proton chemical shift, J-coupling and concentration values reported by Govindaraju et al. 2 and Kraiser et al. 3 . A 2D J-resolved PRESS4 sequence was implemented in General Approach to Magnetic Resonance Mathematical Analysis (GAMMA) 5 to create 1H-MRS for 64 different TE’s (minTE=35ms, ΔTE=2.5ms, 64 steps, 5000Hz sweepwidth, 2048 points). Monte Carlo simulations were performed to estimate the optimal TE combinations for best Glu and mI detection in MATLAB (The Mathworks Inc., Natick, MA) 6. 1H-MRSI of GE Braino phantom and a healthy volunteer were acquired at 3T Siemens Prisma scanner using 10 different echo times that were determined by using Monte Carlo simulations (TR=5000 ms, TE=35, 37, 42, 47, 50, 52, 70, 77, 147, 162 ms, 1000 Hz, 1024 points, FOV=120x120 mm, NSA=1, 16x16 array, total scan time=60min). The raw 1H-MR spectral data of each TE was exported oflline, and apodization and water removal were applied using jMRUI 5.2 (http://www.jmrui.eu), before TE-averaging. Keeping clinical scan time limitations in mind, selected subsets of 2, 3, 4 and 5 TE’s were used to create TE-averaged PRESS spectra of both phantom and healthy volunteer data. The area under the peak was estimated for choline (Cho, 3.2 ppm), creatine (Cr, 3 ppm), Glu (2.35 ppm), glutamine (Gln, 2.43 ppm), mI (3.52 ppm) and N-acetyl aspartate (NAA, 2 ppm) using an in-house program written in MATLAB. Metabolite peak intensities were then normalized by the Cr intensity of that voxel. The percent coefficient of variation (CV) was calculated as 100 * standard deviation (std)/mean for each peak of every combination result of phantom data.Results
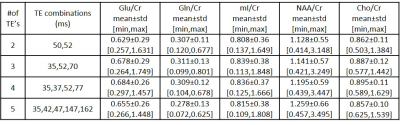
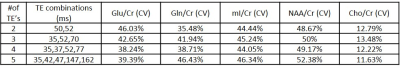
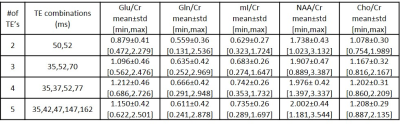
Figures 1 and 2 show 35ms PRESS (a) and optimized TE-averaged PRESS results for 2,3,4 and 5 TE combinations (b-e) for the phantom and volunteer data, respectively. For all TE combinations, Glu metabolite peak was clearly separable from glutamine (Gln) at around 2.35 ppm and mI metabolite peak was observed at around 3.52 ppm in phantom data. The combination of four TE’s qualitatively had the most prominent Glu peak and visible mI peak in volunteer data. Highest Glu/Cr and mI/Cr for phantom data were observed in 4 and 3 TE combinations, respectively (Table 1). Cho/Cr had the lowest (11.63%-13.48%), and NAA/Cr had the highest (48.67%-52.38%) %CV in all TE combinations for the phantom data (Table 2). Glu/Cr and mI/Cr had the lowest %CV (38.24% and 44.05%, respectively) at 4 TE combinations for phantom data. Highest Glu/Cr and mI/Cr were observed in the combination of 4 TE’s in volunteer data (Table 3).Discussion
Routine 1H-MRSI techniques could not reliably yield unobstructed Glu and Gln, peaks, which is a limitation for diagnosis, grading and follow-up of some brain abnormalities. Although TE-averaged PRESS enables separate quantification of Glu, Gln and mI metabolites, it requires a long scan time due to the acquisition of large number of TE’s. Our study indicated that optimized TE-averaged PRESS with reduced number of TE’s successfully quantified separate Glu and mI peaks in human brain.Conclusion
Optimization of echo times for TE-averaged PRESS enabled faster and unobstructed quantification of Glu, Gln and mI metabolites in human brain at 3T. In future studies, compressed sensing will be implemented for accelerating 2D J-resolved PRESS for further reducing the scan time of TE-averaged PRESS.Acknowledgements
This study is supported by Bogazici University BAP grant 10844SUP and 13260D.References
1. Hurd, R., et al., Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med, 2004. 51(3): p. 435-40.
2. Govindaraju, V., K. Young, and A.A. Maudsley, Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed, 2000. 13(3): p. 129-53.
3. Kaiser, L.G., et al., A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed, 2008. 21(1): p. 22-32.
4. Ryner, L.N., J.A. Sorenson, and M.A. Thomas, Localized 2D J-resolved 1H MR spectroscopy: strong coupling effects in vitro and in vivo. Magn Reson Imaging, 1995. 13(6): p. 853-69.
5. Smith, S.A., et al., Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. Journal of Magnetic Resonance, Series A, 1994. 106(1): p. 75-105.
6. Hatay, G.H. and E. Ozturk-Isik. Optimization of Echo Times for TE-Averaged PRESS Spectral Editing Technique Using Monte Carlo Simulations. in European Society for Magnetic Resonance in Medicine and Biology (ESMRMB) 2017. Barcelona, Spain.
Figures