1294
Accelerated Correlated Spectroscopic Imaging in Two Spectral-Three Spatial Dimensions with Slice-selective Adiabatic Refocusing Pulses in Human Calf Muscles1Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 3Greater Los Angeles Veterans Affairs Medical Center, Los Angeles, CA, United States, 4School of Nursing, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
Synopsis
An optimized version of the five-dimensional (5D) echo-planar correlated spectroscopic sequence using an adiabatic full passage (AFP) RF pulse pair has been implemented on a 3T MRI/MRS scanner equipped with a 15-channel transmit/receive coil. The sequence was initially tested using a corn oil phantom. The calf muscle of twelve healthy subjects (age 27.5±3.1 years) and six diabetic type 2 subjects was studied (age 62.3±9.8 years). The AFP pulse pair enabled a sharper profile and minimal chemical shift misregistration. The localization of the volume of interest showed differential distribution of metabolites and lipids in human calf muscle and tibial marrow.
Introduction:
Echo-planar correlated spectroscopic imaging (EP-COSI)1, which combines L-COSY2 with an echo-planar (EPI)3 readout for correlated SI, acquires better-resolved two dimensional (2D) spectra from multiple spatial regions and has been implemented in rat brain1 and in humans4,5. Recently, using accelerated technique based on compressed sensing, EP-COSI has been extended to acquire five dimensional (3 spatial and 2 spectral dimensions) EP-COSI data6 in human calf muscle. Like other point-resolved spectroscopy (PRESS) based in vivo SI techniques with conventional pulses, EP-COSI also suffers from large chemical shift displacement error (CSDE), non-uniform refocusing, and spatially dependent magnetization transfer resulting in reduced cross peak intensity. It has been demonstrated that using adiabatic selective refocusing pulses with relatively higher bandwidth, these artifacts can be minimized7,8. Adiabatic localization has been incorporated into COSY sequences9-11. Here, we present an enhanced version of accelerated 5D EP-COSI by introducing adiabatic radiofrequency pulses for localization along the refocusing dimension and preliminary results in human calf muscle in vivo. We hypothesize that adiabatic pulses will improve slice selection profile and reduce chemical shift artifacts along the refocusing dimension.Materials and Methods:
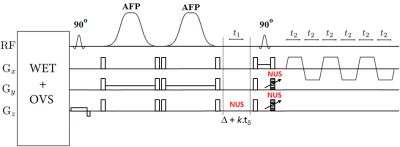
The standard 5D EP-COSI sequence which uses a 90°–180°-Δt1-90° scheme for localization was modified by employing a pairs of adiabatic pulses (AFP) in place of the 180° pulse (Fig. 1). A non-adiabatic, optimized slice-selective 90° excitation pulse as well as the slice selective 90° before the bipolar EPSI read-out trains was still used. In this way, only a pair of AFP pulses was used to keep the RF power within the SAR limits and also TE relatively short. A corn oil phantom was used for acquiring 10 in vitro measurements. The sequence was tested and implemented in the calf muscle of twelve healthy volunteers (age 27.5±3.1 years) and six diabetic type 2 patients (age 62.3±9.8 years). All data were collected on a 3T Prisma MRI scanner using a 15 channel knee ‘transmit/receive’ coil. The following parameters were used for acquiring the 5D NUS- based EP-COSI phantom data: TR/TE = 2s/30 ms, voxel resolution=3.37cm3, 64 Δt1 increments, 512 bipolar echo pair, FOV= 24x24x12cm3, F1 and F2 bandwidths of 1250 Hz and 1190 Hz respectively. A non-water-suppressed EP-COSI data with t1=1 was also recorded. For in-vivo NUS data, TR was 1.5s with scan time ~26min. Other scan parameters were the same as that of the phantom. Acquired data were extracted, reconstructed and post-processed6 with a library of custom MATLAB-based program.Results:
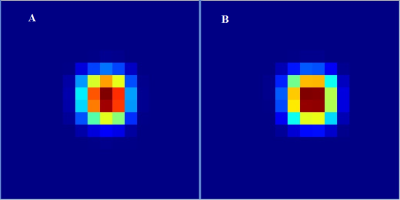
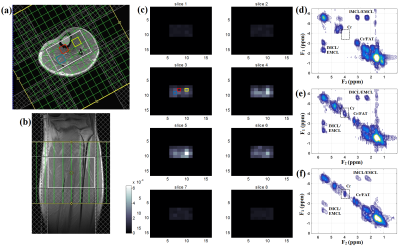
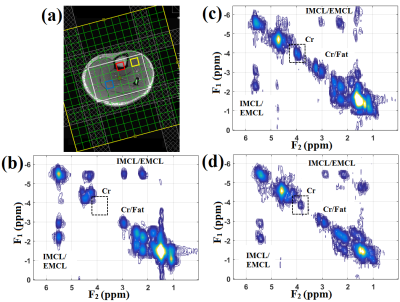
Figure 2 shows the comparison of slice profile of the 2D diagonal peak of creatine (Cr) at 3.0 ppm for EP-COSI with and without adiabatic pulses from a corn oil phantom experiment. In adiabatic based EP-COSI the peak was localized within the PRESS excitation volume better with minimal leakage and the slice profile was more homogeneous indicating better excitation. In vivo human calf muscle spectra from 5D NUS-EP-COSI using the adiabatic pulse acquired in a 62 years diabetic patient can be seen in Figure 3 from the bone marrow (d), tibias anterior (e) and the soleus muscle (f). The absence of the Cr3.9 peak in the marrow was expected and shows that the spatial information is preserved using the adiabatic EP-COSI. It also preserves features such as the residual dipolar coupling of creatine in the tibialis anterior. The Cr (3.9 ppm) spatial profile for all the slices are shown in Figure 3(c). Fig, 3(a) and (b) show axial and coronal MRI localization of the volume of interest. Fig. 4 shows the results from the 5D adiabatic NUS-EP-COSI of a 35 years healthy volunteer showing spectra from bone marrow (b), tibias anterior (c) and the soleus muscle (d).Discussion:
We have implemented the NUS-based 5D EP-COSI with a pair of AFP for localization along one of the slice directions and performed a feasibility study using the corn oil phantom and human calf muscle. The high bandwidth AFP RF pulses used with slice select gradients enabled sharper excitation profiles than conventional Mao RF pulses used for localization. Comparison study on corn phantom showed a homogeneous metabolite profile and sharper excitation in EP-COSI with AFP. This translates to less signal leakage outside the VOI and might also translate to lesser need for outer volume suppression (OVS) to eliminate extraneous signal. This will also improve peak volume resulting in accurate quantification. There are still few limitations to the work including extending adiabatic half passage (AHP) for other slice localization directions, but this may result in longer TE and higher SAR.Conclusion:
The 5D EP-COSI sequence with AFP offers a sharper slice selection profile, and reduction of chemical shift artifacts along the refocusing dimension.Acknowledgements
This research was supported by grants from 1) NIH/NIBIB (5R21EB020883-02) and 2) NINDS 1R21-NS090956.References
1. Mayer D, Dreher W, Leibfritz D. Fast echo planar based correlation peak imaging: Demonstration on the rat brain in vivo. Magn Reson Med 2000;44(1):23-28.
2. Thomas MA, Yue K, Binesh N, et al. Localized two-dimensional shift correlated MR spectroscopy of human brain. Magn Reson Med 2001;46(1):58-67.
3. Posse S, DeCarli C, Le Bihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology 1994;192(3):733-738.
4. Lipnick S, Verma G, Ramadan S, et al. Echo planar correlated spectroscopic imaging: Implementation and pilot evaluation in human calf in vivo. Magn Reson Med 2010;64(4):947–956.
5. Andronesi OC, Gagoski BA, Adalsteinsson E, Sorensen AG. Correlation chemical shift imaging with low-power adiabatic pulses and constant-density spiral trajectories. NMR Biomed 2012;25(2):195–209.
6. Wilson NE, Burns BL, Iqbal Z, Thomas MA. Correlated spectroscopic imaging of calf muscle in three spatial dimensions using group sparse reconstruction of undersampled single and multichannel data. Magn Reson Med 2015;74(5):1199-1208.
7. Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 2001;153:155–177.
8. Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1–6.
9. Ramadan S, Mountford CE. Adiabatic localized correlation spectroscopy (AL-COSY): application in muscle and brain. J Magn Reson Imaging 2011; 33(6):1447–1455.
10. Lin M, Kumar A, Yang S. Two-dimensional semi-LASER correlation spectroscopy with well-maintained cross peaks. Magn Reson Med 2014;72(1):26-32.
11. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 2012;4(116):116ra4.
Figures