1292
Flip Angle Corrected Multi-TR, Multi-TE 1H MR SpectroscopyGavin Hamilton1, Alexandra N Schlein1, and Claude B Sirlin1
1Liver Imaging Group, Department of Radiology, University of California, San Diego, La Jolla, CA, United States
Synopsis
Multi-TR, multi-TE 1H MRS estimates T1 and T2 of fat and water and liver proton density fat fraction in a single breath-hold. This approach uses a steady state solution, which assumes a perfect 90° pulse is generated which is not guaranteed in vivo, possibly introducing T1 errors. We introduce a flip angle corrected multi-TR, multi-TE 1H MRS sequence based on a non-steady state approach and demonstrate, in phantoms, that while the multi-TR, multi-TE MRS sequence estimates T1 dependent on the flip angle, the flip angle corrected multi-TR, multi-TE MRS sequence estimates T1 independent of flip angle.
Introduction
In vivo liver multi-TR, multi-TE (MTRTE) 1H MR spectroscopy has previously been used to estimate the T1 and T2 of fat and water and the proton density fat fraction in a single breath-hold1. This approach uses a steady state solution, which assumes that a perfect 90° pulse is generated. For in vivo liver MRS this perfect 90° pulse is not guaranteed, which may introduce errors in the steady state solution. Alternatively, using a non-steady state approach allows the flip angle to be estimated, giving flip angle corrected T1 and T2 estimates. In this study we introduce a flip angle corrected version of the multi-TR, multi-TE 1H MRS sequence (FAC MTRTE) based on a non-steady state approach and compare, in phantoms, the effect of flip angle on estimated T1 by the two sequences.Sequence Design
Both MTRTE and FAC MTRTE acquire multiple STEAM sequences in a single 21 second acquisition. MTRTE acquires 32 spectra, whereas FAC MTRTE acquires 30 spectra. The timings of the two sequences are shown in table 1 and table 2. Whilst MTRTE has pre-pulses (P1-P4) in table 1 to get to equilibrium, these are not required for FAC MTRTE. In FAC MTRTE, there are large changes in consecutive TRs to maximize non-steady conditions allowing estimation of flip angle. To estimate T1 and T2, MTRTE non-linearly fitted the measured peaks area to the signal (Sn) using the standard steady state equation $$S_{n}=M_{0}(1-e^{(-\tau_{n}/T1)})e^{(-TE_{n}/T2)}$$ where τn and TEn are given in table 1. To estimate T1 and T2 for FAC MTRTE, the longitudinal magnetization at each acquisition (Mn) was calculated by $$M_{n}=M_{n-1}e^{(-\tau_{n}/T1)}\cos(\alpha)+M_{0}(1-e^{(-\tau_{n}/T1)})$$ with the signal given by $$S_{n}=M_{n}e^{(-TE_{n}/T2)}\sin(\alpha)$$ where α is the flip angle, M0 the equilibrium longitudinal magnetization, and τn and TEn are given in table 2. The calculated signals for each acquisition are then non-linearly fitted to the measured peak areas. A simulation of the longitudinal magnetization in FAC MTRTE for typical water T1 of 822 ms2 and flip angles 80°, 90° and 100° is shown in figure 1.Methodology
1H MR spectra were acquired at 3 Tesla (GE Signa EXCITE HDxt, GE Healthcare, Waukesha, WI) using a quadrature head coil in a vegetable oil and Gd-doped saline phantom. For both phantoms, the transmitter gain (TG), which determines the amount of power required to generate a true 90° flip, was set automatically by the scanner. Both MTRTE and FAC MTRTE were acquired in the same voxel with the same TG. The TG was then manually varied to simulate the flip angle deviating from 90°. MTRTE and FAC MTRTE were again acquired in the same voxel with the changed TG. This was repeated across a range of TGs chosen to approximately simulate a 70° to 110° flip angle range. The spectra were transfer offline and a single experienced observer analyzed the spectra using the AMARES algorithm3 ) included in the MRUI software package4. T1 and T2 was calculated for water and fat (sum of fat peaks between 0 and 3 ppm) for the doped saline and vegetable oil phantom respectively, and the values were compared between the two sequences.Results
The variation of water T1 with TG for the two sequences is shown in figure 2, and same is shown for fat T1 in figure 3. In both phantoms, the T1 estimated by MTRTE showed large biases with estimated T1 changing linearly with TG (or flip angle). The water T1 changed by 420 ms across the range of TG, and fat T1 changed by 91 ms. In the FAC MTRTE, there was no evidence of a dependence of T1 estimate on flip angle. The range of T1 across the TG range was only 25 ms for water T1 and 5ms for fat T1. There was no difference between the T2 estimates given by the two sequences.Conclusion
We have shown that T1 estimates given by the multi-TR, multi-TE MRS sequence are dependent on the flip angle, whilst the flip angle corrected multi-TR, multi-TE MRS sequence estimates T1 independent of flip angle.Acknowledgements
No acknowledgement found.References
- Hamilton G, Middleton MS, Hooker JC, et al. In vivo breath-hold 1H MRS simultaneous estimation of liver proton density fat fraction, and T1 and T2 of water and fat, with a multi-TR, multi-TE sequence. J Magn Reson Imaging 2015; 42: 1538-1543
- Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed 2011;24:784-790
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35-43
- Naressi A, Couturier C, Devos J, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001;12:141-152
Figures
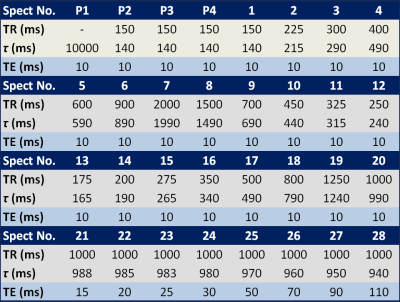
Table 1 Sequence timing of multi-TR, multi-TE 1H MRS
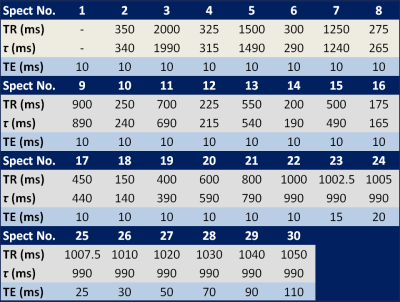
Table 2 Sequence timing of flip angle corrected multi-TR, multi-TE 1H MRS
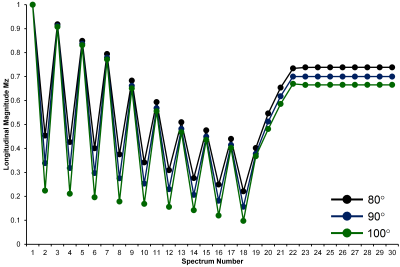
Figure 1 Simulation of the longitudinal magnetization
in FAC MTRTE 1H MRS for typical water T1 of 822 ms at flip angles of 80°, 90° and 100°.
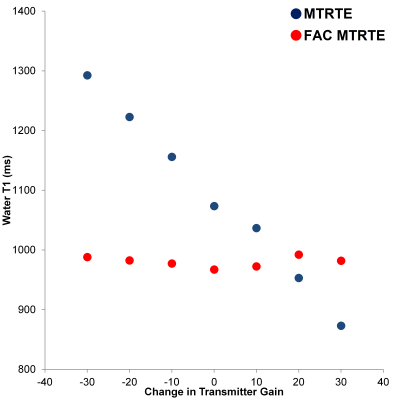
Figure 2 Comparison of the water T1 in a Gd-doped Saline phantom as estimated by FAC MTRTE 1H MRS and MTRTE 1H MRS across a range of TG (flip angles).
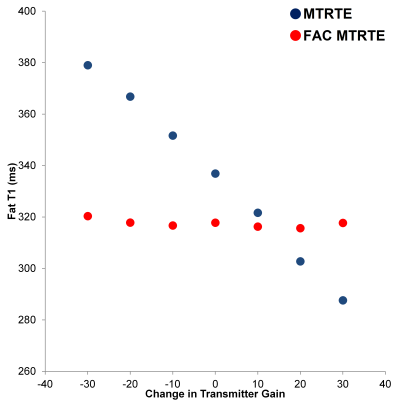
Figure 2 Comparison of the fat T1 is a vegetable oil phantom as estimated by FAC MTRTE 1H MRS and MTRTE 1H MRS across a range of TG (flip angles).