1291
Test-retest reliability of real-time frequency and motion corrected Hadamard encoded spectral editing (CHASE)1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 2Philips, Copenhagen, Denmark, 3Center for Magnetic Resonance, Dept. Electrical Engineering, Technical University of Denmark, Lyngby, Denmark
Synopsis
Inhibitory neurotransmitter GABA and antioxidant GSH are suggested to be implicated in psychiatric and neurological disorders. Because of their relatively weak signals, spectral editing is necessary to assess GABA and GSH in the human brain. Hadamard encoding can be applied simultaneously for spectral editing of GABA and GSH. As both small metabolite signals and Hadamard encoding are highly susceptible to frequency drift and motion, real-time frequency and motion correction significantly improves spectral quality. The data obtained in this study so far suggest good test-retest reliability of real-time frequency and motion corrected Hadamard encoded spectral editing (CHASE) for GABA and GSH.
Purpose
To assess the repeatability of real-time frequency and motion corrected Hadamard encoded spectral editing of gamma-aminobutyric acid (GABA) and glutathione (GSH) in the human brain in vivo.Introduction
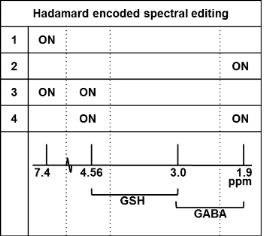
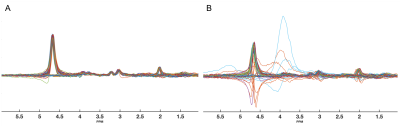
In this study, the repeatability and robustness of CHASE for measurement of GABA and GSH is assessed. The major inhibitory neurotransmitter GABA and the predominant antioxidant GSH are both suggested to be implicated in neuropsychiatric and neurodegenerative diseases. As the signals of both metabolites are relatively weak and overlapped by larger signals of more abundant metabolites, spectral editing is necessary to isolate the signals. In traditional MEGA-editing1 a refocusing pulse is applied to a coupled resonance in the even acquisitions while in the odd acquisitions refocusing is not applied, resulting in an edited spectrum when subtracting the even and odd acquisitions. Using a Hadamard scheme2, multiple small-signal metabolites can be simultaneously assessed, thereby reducing total scan time. The method makes use of the fact that although GABA and GSH both have resonances around 3 ppm, they have coupled resonances at different frequencies, i.e. 1.89 and 4.56 ppm respectively. Instead of a two-step editing scheme, a four-step Hadamard scheme (Fig. 1) is applied using a dual-band refocusing pulse at 1.9 and 4.56 ppm in the fourth step3 However, the Hadamard scheme is vulnerable to motion artefacts and, due to the four step paradigm, four spectra most be discarded for every distorted acquisition when not using real-time frequency and motion correction. Likewise, are the small signals of GABA and GSH highly susceptible to frequency drift and motion. Real-time frequency and motion correction restores signal intensities by reacquiring motion-affected acquisitions and is thus essential for accurate assessment of edited metabolites3 (Fig. 2). The high spectral quality and time-efficiency of real-time frequency and motion corrected Hadamard spectral editing (CHASE) is therefore highly suitable for studies in motion-prone patients.Methods
Participants: Ten healthy volunteers (21-35 years, mean±SD=27±6 years, M/F=5/5) were included in the study thus far. Two were excluded due to failure to show up to one or both sessions and one was excluded due to non-compliance with the motion paradigm. Further analysis was thus based on seven subjects (21-35 years, mean±SD=29±5 years, M/F=4/3). Recruitment is still ongoing and 20 participants in total are expected to be included. Experiments were performed in accordance with the local ethical guidelines. In both sessions, a CHASE sequence was acquired in combination with a motion paradigm. During scanning, participants performed timed motions consisting of four natural movements (sneezing, coughing, deep breathing, nodding); every movement was performed five seconds at a time with twenty seconds between movements.
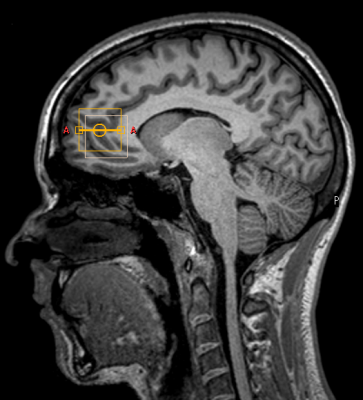
MR acquisition: MRS experiments were performed on a 3T MR scanner (Philips, Best, The Netherlands) in combination with a 32-channel receive head coil. A T1 weighted sequence (288 slices, slice thickness=0.85 mm, TR=6.0 ms, TE=2.7 ms, flip angle=8 degrees, FOV=245x245x208, acquisition matrix=288x188, scan duration=5:43 min) was obtained for anatomical reference. Hadamard encoded spectral editing (TR/TE=2000/80 ms, 128 averages, voxel dimensions = 25x25x25 mm3) (Fig. 1) was performed with volumetric 3D EPI navigators4 (Clinical science software, Philips, Best, The Netherlands) that were interleaved before water suppression in every acquisition5. A frequency drift measurement was performed prior to each 3D EPI volume by readouts of the central k-space line. Reacquisition of an acquisition between two neighbor navigators was performed if a motion update exceeded 1 mm of displacement. All spectroscopy experiments were performed bilaterally in the anterior cingulate cortex (Fig. 3).
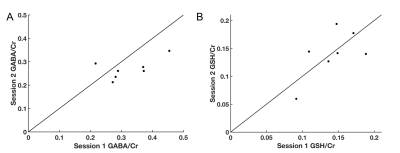
Data analysis: Edited spectra were analysed using Matlab (Matlab 2016a, The MathWprks, Inc., Natick, Massachusetts, United States). Acquisitions with more than 30 datapoints which deviated more than twice the standard deviation from the mean of all other points at the same frequency in a sequence were removed before fitting of the metabolite peaks. Creatine was used as a reference. Statistical analysis was performed in SPSS (SPSS 22.0, Chicago, IL, USA). Test-retest reliability for GABA/Cr and GSH/Cr, was assessed by calculating the intraclass correlation coefficient (ICC) using a two-way mixed model ANOVA.
Results
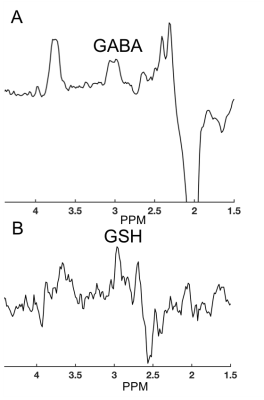
Representative spectra for GABA and GSH CHASE are displayed in Figure 4. Figure 5 shows the measurements obtained per session with CHASE for GABA/Cr and GSH/Cr respectively. The ICC for GABA/Cr was 0.66 (p=0.108) and the ICC for GSH/Cr was 0.76 (p=0.054). All CRLBs were below 20.Discussion
It has previously been shown that CHASE restores signal intensity when this has been compromised by movement3. The data obtained in this study so far suggest good test-retest reliability for GABA and GSH. Subject recruitment is still ongoing and will continue until N=20.Acknowledgements
This research is supported by the Danish Council for Independent Research grant no. 6111-00349A.References
1. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed, 1998, 11:266-272.
2. Saleh MG, Oeltzschner G, Chan KL, Puts NA, Mikkelsen M, Schär M, Harris AD, Edden RA. Simultaneous edited MRS of GABA and glutathione. Neuroimage, 2016, 142:576-582.
3. Marsman A, Boer VO, Andersen M, Petersen ET. Real-time frequency and motion corrected Hadamard encoded spectral editing (CHASE). Proc Intl Soc Magn Reson Med, 2017, p.5943.
4. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, Van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med, 2012, 68:389-399.
5. Henningsson M, Mens G, Koken P, Smink J, Bornar RM. A New framework for interleaved scanning in cardiovascular MR: Application to image-based respiratory motion correction in cornary MR angiography. Magn Reson Med, 2015, 73:692-696.
Figures