1287
High resolution mapping of GABA+ and Glx using motion-corrected, spiral-accelerated, edited 1D-semiLASER MRSI in the human brain at 7T1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 4Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 6Fetal-Neonatal Neuroimaging; Developmental Science Center, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 7Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
Synopsis
In vivo detection of gamma-aminobutyric acid (GABA) and glutamate (Glu), both major neurotransmitters in the human brain, benefits from the higher sensitivity and SNR at ultra-high field (7T) compared to lower field strengths. However, strong B1+ inhomogeneities and chemical shift displacement errors, as well as subject motion and carrier frequency drifts can significantly impair the experiment. We preliminarily propose the first high resolution full-slice in vivo mapping of GABA+ at 7T. Combining spatial-spectral spiral encoding for MRSI acceleration with B1-insensitive adiabatic pulses and real-time motion correction allows unprecedented high resolution J-difference editing at 7T in comparably short scan time.
Introduction
In vivo detection of gamma-aminobutyric acid (GABA) and glutamate (Glu), both major neurotransmitters in the human brain, benefits from the higher sensitivity and SNR at ultra-high field (7T) compared to lower field strengths [1]. Due to the low abundance of GABA and its spectral overlap with more abundant metabolites (e.g. creatine), unconventional methods need to be employed to extract the small signal. Among different approaches J-difference editing is quoted to have the highest SNR [2]. However, spectral quality at 7T is often limited by, first, strong B1+ inhomogeneities and chemical shift displacement errors [3]. The use of B1+-insensitive adiabatic pulses has been proposed to mitigate this challenge [4]. Second, subject motion and scanner instabilities result in subtraction artifacts that may prevent quantification of GABA+ (GABA plus co-edited macromolecules) and Glx (glutamate plus co-edited glutamine) [5,6]. Motion-control methods using interleaved image-based navigators have been proposed [7] and have demonstrated to increase data quality by real-time updating the MRSI sequence [8]. Up to now, GABA+ detection was limited by spatial coverage. Besides single-voxel spectroscopy [9,10], only few single slice 2D-MRSI and 3D-MRSI studies have been published [11,12,13]. However, all suffered from rather low inplane resolution together with large slice/slab thicknesses making the assessment of spatial GABA+ distribution challenging. We preliminarily propose the first high resolution full-slice in vivo mapping of GABA+ at 7T. Combining spatial-spectral spiral encoding for MRSI acceleration with B1+-insensitive adiabatic pulses and real-time motion correction allows unprecedented high resolution J-difference editing at 7T in comparably short scan time.Methods
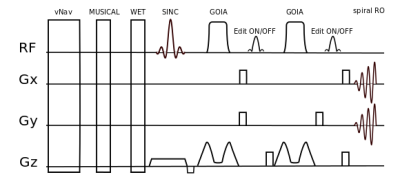
Phantom and in vivo measurements were performed on a Siemens Magnetom 7T whole-body MR scanner using a 32-channel receive coil array combined with a volume transmit coil. MUSICAL [14] was adapted to be applied with spiral-encoding for proper coil combination. Slice excitation was achieved by 1D-semiLASER using a 900us slice selective SINC excitation pulse and one pair of B1+-insensitive adiabatic GOIA pulses (W16,4 modulation, 8ms, 10kHz) refocusing a 16mm slice [8]. Cosine-filtered Gaussian editing pulses (9.8ms, 140Hz BW) were inserted into the sequence (Fig.1). EDIT-ON (1.9ppm) and EDIT-OFF (7.5ppm) spectra were acquired in an interleaved fashion to reduce subtraction artifacts. Spoiler gradients (30mT/m amplitude, 4ms duration) were arranged around the editing pulses as initially proposed by Mescher et al. [15]. One CHESS fat-sat pulse was incorporated into the WET water suppression schema to reduce extracranial lipid artifacts. TE/TR were 68ms/1.9s. Constant density spiral-encoding in the (kx,ky)-plane was used (3 temporal, 8 angular interleaves) [16]. The FOV of 220x220mm² was subdivided into 31x31 voxels (interpolated to 32×32). MRSI data were obtained with 1024 spectral points, 2702Hz spectral bandwidth, 10 averages, 2-step phase cycling, 77° FA and TA 15:25min. Real-time correction was performed using dual-contrast volumetric EPI navigators (vNavs) inserted prior to WET. For each TR the vNavs determine the required rotation and translation of the VOI and allow online updating before the subsequent MRSI excitation. A 7T optimized vNav protocol was employed, including water excitation and advanced phase correction [8]. Phantom (20mmol/l GABA) measurements were performed to ensure the functionality of the J-difference editing scheme. Resonance shapes from ON/OFF/DIFF spectra were qualitatively compared to simulated data created with NMRScope-B. Data was post-processed with an in-house pipeline [17] including Hamming filtering and 8Hz exponential filtering. Voxels were fitted using LCModel to create metabolic maps.Results
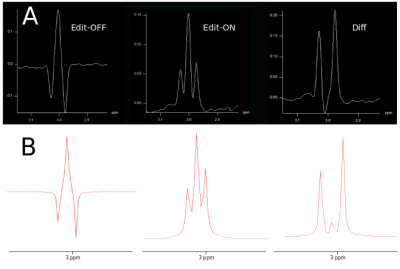
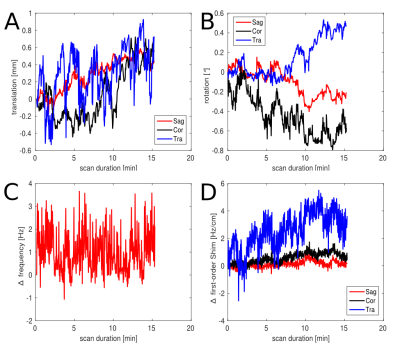
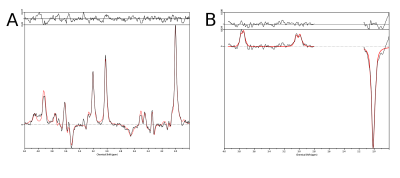
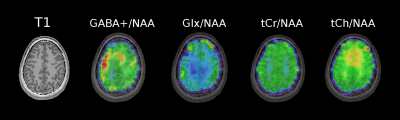
Phantom measurements (5Hz line width) proved the correct spectral behavior of the 3.02ppm GABA resonance during EDIT-ON/OFF/DIFF compared to simulated spectra (Fig.2). Fig.3. depicts the tracking of the VOI position, carrier frequency and 1-st order B0 shim terms during an in vivo scan based on which real-time updates were applied. From one in vivo measurement sample OFF and DIFF spectra are shown in Fig. 4. Fig. 5 depicts metabolic maps (ratios to NAA) of GABA+ and Glx obtained from DIFF spectra, as well as tCr and tCh from OFF spectra.Discussion/Conclusion
This preliminary study demonstrated that using J-difference editing in a 1D-semiLASER sequence at 7T is feasible and allows unprecedented, high resolution mapping of low-abundant metabolites, such as GABA+. Subject motion was successfully tracked and real-time updated using volumetric navigators. Strong B1+ differences across the brain slice reduce the editing efficiency. Ongoing work deals with designing a refocusing MEGA pulse for improved B0 and B1 inhomogeneity handling at 7T, which is also compatible with an echo time of 68ms. Next steps will also include extending this sequence to 3D and, thus, performing metabolic mapping of a volume covering a larger part of the whole human brain at 7T, as well as targeting other interesting metabolites such as GSH and 2HG.Acknowledgements
This study was supported by the FFG Bridge Early Stage Grant No. 846505, FWF Grant KLI 646 and FWF Grant P 30701.References
[1] Ganji SK., et al., 2014, NMR Biomed. 27(10):1167-75
[2] Puts NA., et al., 2012, Prog. Nucl. Magn. Reson. Spectrosc. 60, 29–41
[3] Truong TK., et al., Proc. ISMRM 2170
[4] Hess AT., et al., 2012, NMR Biomed, 25(2):347-58
[5] Edden RA., et al., 2007, Magn. Reson. Med. 58, 1276–1282
[6] Heckova E., et al., 2017, Radiology. Sep 28:170744
[7] Bogner W., et al., 2014, NeuroImage, 88:22-31
[8] Moser P., et al., Proc. ISMRM 2017, Nr. 1253
[9] Gao F., et al., 2013, NeuroImage 78, 75–82
[10] Mullins RA., et al., 2014, NeuroImage 1, 43–52
[11] Zhu H., et al., 2011, Magn. Reson. Med. 65, 603–609
[12] Choi IY., et al., 2006, NeuroImage 33, 85–93
[13] Bogner W., et al., 2014, NeuroImage, 103:290-302
[14] Strasser B., et al., 2013, NMR Biomed, 26(12):1796-805
[15] Mescher M., et al., 1998, NMR Biomed, 11, 266–272
[16] Adalsteinsson E., et al., 1998, Magn Reson Med, 39:889–898
[17] Považan M., et al., 2014, Proc. 2nd TRANSACT Meeting, Bern, p. S2
Figures