1276
Cross-vendor standardization of a 3 T MRS protocol with semi-LASER1Russell H. Morgan Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Center for Magnetic Resonance Reseach, University of Minnesota, Minneapolis, MN, United States
Synopsis
Acceptance of 1H-MRS for clinical use is hindered by variability in methodology across platforms. Cross-vendor standardization is thus desirable for large-scale studies to be conducted. Here, we standardize a semi-LASER scheme (TE=30 ms) with identical pulses, inter-pulse durations and acquisition protocol in phantom and healthy volunteers on Philips and Siemens 3 T systems. The implemented method resulted in high quality spectra with matched SNR, linewidth and spectral patterns in phantom and similar estimated metabolite concentrations in vivo: between-subject CVs for NAA were (2.6-11.0)% and (3.3-10.2)% for Philips and Siemens, respectively. This method highlights the potential for pooling data across multiple sites.
Aim
To standardize a semi-LASER MRS acquisition strategy across vendors and compare implementations in phantom and healthy volunteers.Introduction
Wide clinical acceptance of MRS is dependent on reliable acquisition across sites and vendors. A recent consensus effort established the need for standardized protocols,1 which would allow multi-site, cross-platform studies on large patient cohorts. Previous studies have indicated variability in measured metabolite concentrations 2,3 and sites and vendors vary in their implementation of MRS methods.4 Deelchand et al. have shown that a standardized acquisition within vendor resulted in good agreement (coefficients of variation, CVs = 6-12%).5 However, differences in hardware performance, sequence parameters and methodology pose a challenge for cross-vendor standardization. Localization with semi-LASER 6,7 provides robust MRS measurement, with reduced chemical shift displacement and insensitivity to B1 inhomogeneity,8 and matched adiabatic refocusing has previously been demonstrated across vendors.9 In this study, we implement the same semi-LASER schemes at 3 T on Siemens and Philips platforms, with identical sequence parameters and protocol, to assess the potential for reliable cross-vendor MRS acquisition.Methods
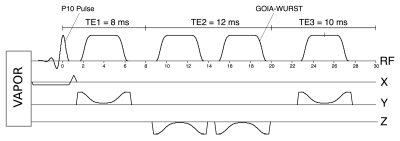
Standardization was carried out on Philips Achieva and Siemens Prisma 3 T MR systems equipped with 32-channel head coils. Identical implementations of semi-LASER were achieved (Fig. 1) with P10 excitation pulses (duration = 2.6 ms) and refocusing using GOIA-WURST pulses 10 (duration = 4.5ms, BW = 10 kHz, HS16), which have clean inversion profiles at a peak B1 of 15 μT.9 A short-TE scheme, with matched inter-pulse durations (TE = 30 ms; TE1/TE2/TE3 = 8/12/10 ms), was implemented on each vendor and water suppression was performed with VAPOR.11 Spectra were acquired on both vendors from a standard ‘Braino’ phantom (General Electric, Milwaukee, WI) with NT = 64, TR = 3 s, SW = 6 kHz and samples = 2048. First and second order shimming was performed using a system shim. Raw spectra were coil-combined and post-processed using similar analysis pipelines. Voxel placement was guided with template images. Spectra were obtained from 5 healthy age-matched volunteers on each system (Philips: age = 37±14, 2 F; Siemens: age = 34±12, 1 F) from pons (16x16x16 mm3), cerebellar white matter (CBWM) (17x17x17 mm3) and putamen (10x25x11 mm312) with NT = 64 and TR = 5 s. The ‘LCModel’ program 12 was used to fit the spectra using an identical basis set of 21 simulated metabolites including measured macromolecular spectrum. SNR was measured as ratio of tCho amplitude to RMS noise in unfiltered spectra and water suppression was determined by the ratio of residual to unsuppressed water. Metabolite differences were assessed using an unpaired two-tailed t-test with Bonferroni correction (significance threshold: α=0.05/21).Results
Averaged
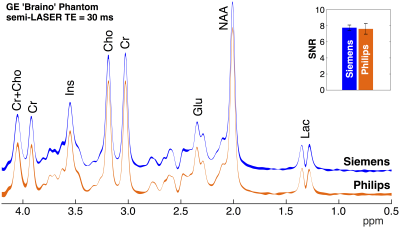
spectra over three acquisitions in Braino phantom are shown in Fig. 2.
Remarkably similar spectral profiles were observed between vendors. The SNR was
calculated to be 7.6±0.7 and 7.8±0.3 on Philips and Siemens platforms
(p=0.75), respectively. Mean LCModel linewidth
estimates were 2.0 Hz for Siemens and 1.8 Hz for Philips (p=0.87). Representative in
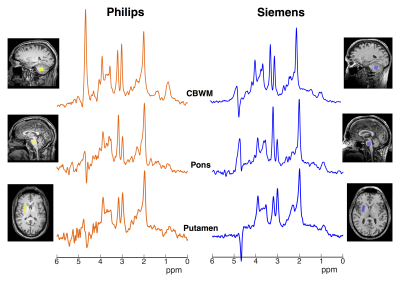
vivo spectra acquired from two volunteers (Philips: 54 M; Siemens: 51 M)
are shown in Fig. 3 with residual water peak. High quality spectra were
obtained on both vendors with minimal baseline artefact. Some spectra displayed
presence of unwanted coherences (i.e. putamen in Fig. 3). Across all subjects,
adequate water suppression was achieved, although water suppression efficiency was
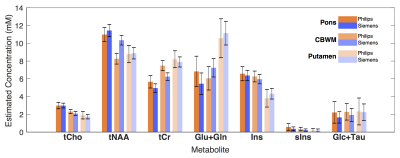
higher on Siemens (99.6%) than Philips (98.1%) (p=0.004). Mean metabolite concentrations over all subjects (Fig. 4)
were matched between vendors for all major metabolites (after Bonferroni
correction). The largest differences were observed in CBWM voxel for tNAA (p=0.003) and tCr (p=0.006) (visible in Fig. 3). Between-subject CVs for tNAA
concentration across voxels ranged from (2.6-11.0)% and (3.3-10.2)% for Philips
and Siemens, respectively.Discussion/Conclusion
This study demonstrates the potential of a standardized acquisition for high-quality, semi-automated single-voxel MRS acquisition across vendors. Identical spectral profiles obtained in phantom, with matched linewidth and SNR, highlight the similarity of the standardized semi-LASER implementation on both platforms. In vivo acquisition also resulted in comparable concentrations across major metabolites, with similar low within-site CVs (< 11 % for tNAA). Differences in cerebellar concentrations may be due to white matter differences in voxel content arising from manual VOI placement. Automating placement using atlas-based registration may reduce cross-site variability in future studies.13,14 Although the mean age of participants was matched, inter-individual differences may lead to variation with the given sample size. Future work will aim to refine this standardization effort, including matched OVS and crusher gradients to eliminate unwanted coherences. Finally, pooling of data between sites, using this method, will enable more efficient and powerful investigation of clinical populations.Acknowledgements
The authors would like to acknowledge the following funding sources: P41 EB015894, P30 NS076408, and R01 NS080816.
References
1. Öz, G. et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 270, 658–679 (2014).
2. Komoroski, R. A. et al. Brain metabolite concentration ratios in vivo: Multisite reproducibility by single-voxel 1H MR spectroscopy. Magn. Reson. Imaging 22, 721–725 (2004).
3. Keevil, S. F. et al. Absolute metabolite quantification by in vivo NMR spectroscopy: II. A multicentre trial of protocols for in vivo localised proton studies of human brain. Magn. Reson. Imaging 16, 1093–1106 (1998).
4. Mikkelsen, M. et al. Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage 159, 32–45 (2017).
5. Deelchand, D. K. et al. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn. Reson. Med. 73, 1718–1725 (2015).
6. Öz, G. & Tkáč, I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn. Reson. Med. 65, 901–910 (2011).
7. Scheenen, T. W. J., Klomp, D. W. J., Wijnen, J. P. & Heerschap, A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn. Reson. Med. 59, 1–6 (2008).
8. Garwood, M. & DelaBarre, L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J. Magn. Reson. 153, 155–177 (2001).
9. Deelchand, D. K. et al. Across-vendor standardization of semi-LASER for single-voxel MRS at 3 Tesla. in ISMRM Workshop on MRS (2016).
10. Andronesi, O. C. et al. Spectroscopic imaging with improved gradient modulated constant adiabaticity pulses on high-field clinical scanners. J. Magn. Reson. 203, 283–293 (2010).
11. Tkáč, I. et al. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 41, 649–656 (1999).
12. Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679 (1993).
13. Park, Y. W. et al. Fast automatic voxel positioning with non-rigid registrations for improved between subject consistency in MRS. in 24th Scientific Meeting of ISMRM (2016).
14. Park, Y. W. et al. In-vivo testing of automatic voxel prescription for high inter-subject reproducibility in single voxel MR spectroscopy. in 25th Scientific Meeting of ISMRM (2017).
Figures