1271
Metabolite cycled density-weighted concentric rings k-space trajectory (DW-CRT) enables 1H magnetic resonance spectroscopic imaging at 3 Tesla in a clinically feasible timeframeAdam Steel1,2, Mark Chiew3, Peter Jezzard4, Natalie Voets4, Puneet Plaha4, M. Albert Thomas5, Charlotte J Stagg4, and Uzay E Emir6
1Nuffield Department of Medicine, University of Oxford, Headington, United Kingdom, 2National Institute of Mental Health, National Institutes of Health, Bethesda, DC, United States, 3Nuffield Department of Clinical Neuroscience, University of Oxford, Headington, United Kingdom, 4Nuffield Department of Clinical Neurosciences, University of Oxford, Headington, United Kingdom, 5Department of Radiology, University of California Los Angelas, Los Angeles, CA, United States, 6School of Health Sciences, Purdue University, West Lafayette, IN, United States
Synopsis
In this study, we demonstrate that a metabolite-cycled semi-LASER pulse localization with density-weighted concentric rings trajectory (DW-CRT) enables high-resolution MRSI to be acquired at 3 Tesla within a clinically feasible acquisition time. High-resolution (5 x 5 x 10 mm3) DW-CRT feasibility at 3T was assessed in 6 healthy volunteers. Subsequently, the clinical utility of this approach was demonstrated by mapping the presence of 2-HG in a patient with a grade III oligodendroglioma tumor.
Introduction
Magnetic resonance spectroscopic imaging (MRSI) is an appealing technique in both research and clinical settings. However, the utility of MRSI has been hampered by long acquisition times, and hardware- and subject-related artifacts [1]. Advances in MRSI acquisition, like DW-CRT and metabolite cycling [2-4], and preprocessing [3, 5, 6] have overcome some of these issues. However, these techniques have not been combined, nor have they been demonstrated at widely available field-strengths. We sought to combine metabolite-cycled DW-CRT with semi-LASER pulse localization with advanced preprocessing to achieve high-quality MRSI within a clinically-feasible timescale and demonstrate the clinical utility by mapping 2-hydroxyglutarate (2-HG) in a patient with a high-grade brain tumor [7-9].Methods
Six healthy volunteers and one patient were scanned in this study. Data were collected using a whole-body 3T Prisma MR system (Siemens, Erlangen) with a 32-channel head coil. GRESHIM was used for B0 shimming [10]. Two asymmetric narrow transition-band adiabatic RF pulses with mirrored inversion profiles were applied in alternate scans to acquire the upfield and downfield (relative to water) spectral resonances before the semi-LASER localization [11, 12]. For metabolite-cycling, an 80 Hz transition bandwidth (-0.95 < Mz/M0 < 0.95) and 820 Hz inversion bandwidth (-1 < Mz/M0 < -0.95), 70 to -750 Hz) downfield/upfield from the carrier frequency (carrier frequency offset=+60 Hz and -60 Hz for downfield and upfield). The sequence used the following adiabatic pulse parameters: hyperbolic secant pulse, HS1/2, with R=10 and 0.9 x Tp, tanh/tan pulse with R=40 and 0.1 x TP) [2]. DW-CRT was prescribed using a Hanning-window [2] and the following parameters: points-per-ring=64, temporal samples=512, resolution=5x5x10 mm3, Rings=24, FOV=240x240x10 mm, TR=1350 ms, TE=32 ms, interleaves=4, timeacquire=13.5 min. For the tumor patient, the TE was changed to 110 ms to be sensitive to 2-HG [7]. Prior to LCModel fitting [13], combined upfield/downfield FIDs were used to remove residual eddy current effects [14] and to combine the phased array coil spectra [15]. Hankel-Lanczos single value decomposition (HLSVD) algorithm filtered any remaining water peak [6], and a lipid-basis penalty was used to remove lipid contamination [16]. To evaluate tumor abnormalities, 2-hydroxyglutarate (2-HG), N-acetyl-aspartate (NAA), and choline (Cho) in affected and unaffected tissue were assessed, and a tumor mask was constructed for the single patient (female, age=72 years; biopsy confirmed IDH-mutant oligodendroglioma). Equivalent voxels were sampled on the unaffected hemisphere.Results
The acquisition and preprocessing enabled construction of high-resolution metabolite maps (Figure 1, Cramer-Rao Lower Bounds in Figure 2). Across all subjects, the median voxel-wise SNR was 18 (interquartile range=14-22) and linewidth was 7.26 Hz (interquartile range=6.16-9.733 Hz; Figure 3, lower). The tumor region from the patient showed abnormal 2-HG, Cho, and NAA Figure 4. As would be expected, the tumor had decreased NAA and increased choline compared with the unaffected hemisphere. In addition, 2-HG, a specific tumor marker, was present within the tumor but was virtually absent on the contralateral side.Conclusions
We demonstrated a DW-CRT semi-LASER sequence with metabolite cycling enables high-resolution metabolite maps to be produced at 3T in a clinically viable acquisition time. In addition to its robustness in healthy volunteers, we demonstrate this technique’s sensitivity to abnormalities in a high-grade tumor patient. Future work should integrate outer-volume suppression to reduce lipid contamination during acquisition.Acknowledgements
No acknowledgement found.References
- Zhu, H. and P.B. Barker, MR spectroscopy and spectroscopic imaging of the brain. Methods Mol Biol, 2011. 711: p. 203-26.
- Emir, U.E., et al., Non-water-suppressed short-echo-time magnetic resonance spectroscopic imaging using a concentric ring k-space trajectory. NMR Biomed, 2017.
- Hock, A., et al., Non-water-suppressed proton MR spectroscopy improves spectral quality in the human spinal cord. Magn Reson Med, 2013. 69(5): p. 1253-60.
- Chiew, M., et al., Density-weighted concentric rings k-space trajectory for 1 H magnetic resonance spectroscopic imaging at 7 T. NMR Biomed, 2017.
- Bilgic, B., et al., Fast image reconstruction with L2-regularization. J Magn Reson Imaging, 2014. 40(1): p. 181-91.
- Cabanes, E., et al., Optimization of residual water signal removal by HLSVD on simulated short echo time proton MR spectra of the human brain. J Magn Reson, 2001. 150(2): p. 116-25.
- Berrington, A., et al., Improved localisation for 2-hydroxyglutarate detection at 3T using long-TE semi-LASER. Tomography, 2016. 2(2): p. 94-105.
- Emir, U.E., et al., Noninvasive Quantification of 2-Hydroxyglutarate in Human Gliomas with IDH1 and IDH2 Mutations. Cancer Res, 2016. 76(1): p. 43-9.
- Pope, W.B., et al., Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol, 2012. 107(1): p. 197-205.
- Shah, S., et al., Rapid Fieldmap Estimation for Cardiac Shimming, in Proceedings 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine. 2009: Honolulu p. 565.
- Scheenen, T.W., et al., Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med, 2008. 59(1): p. 1-6.
- Scheenen, T.W., A. Heerschap, and D.W. Klomp, Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. MAGMA, 2008. 21(1-2): p. 95-101.
- Provencher, S.W., Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed, 2001. 14(4): p. 260-4.
- Klose, U., In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med, 1990. 14(1): p. 26-30.
- Walsh, D.O., A.F. Gmitro, and M.W. Marcellin, Adaptive reconstruction of phased array MR imagery. Magn Reson Med, 2000. 43(5): p. 682-90.
- Lee, J. and E. Adalsteinsson, Reconstruction with High-Resolution Spatial Priors for Improved Lipid Suppression, in Proceedings 18th Scientific Meeting, International Society for Magnetic Resonance in Medicine. 2010: Stockholm p. 965.
Figures
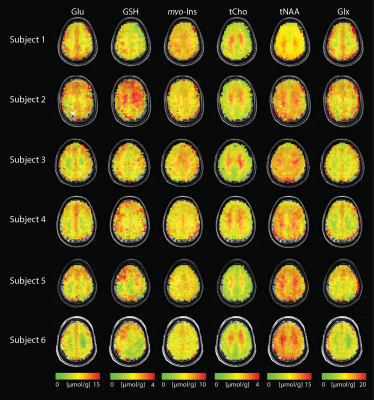
Figure 1.
High-resolution metabolite distribution maps obtained using DW-CRT from
all subjects. tNAA, tCho, Glu, myo-Ins,
GSH, and Glx Concentrations are shown with respect to tCr. Images are shown in neurological orientation.
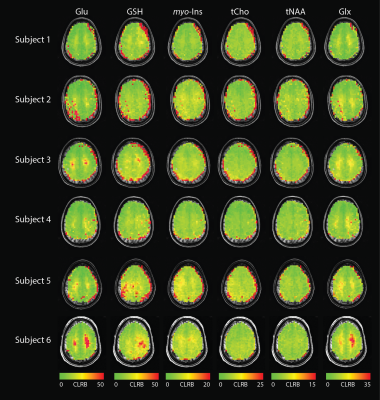
Figure 2. CRLB maps
for tNAA, tCho, Glu, myo-Ins,
GSH, Glx are overlaid on each subject’s anatomical image. Images are shown in neurological orientation.
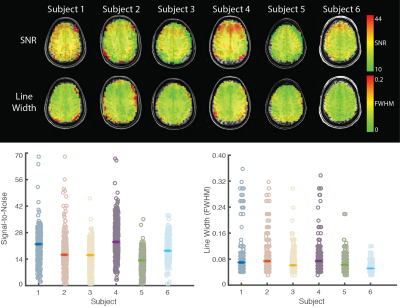
Figure 3. Spatial distribution of signal to noise ratio and
line width across the spectroscopic image.
Upper. In all subjects, both signal to noise and spectral linewidth were
uniformly distributed across the image.
Lower. Voxel-wise signal to noise (left) and linewidth (right) are
shown. Hashes indicate subject mean.
Line-width is presented in parts per million.
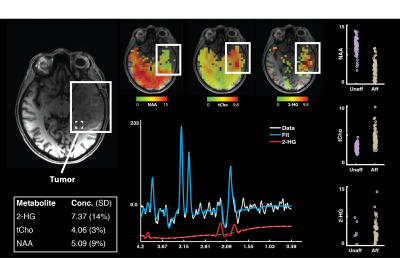
Figure 4. Whole-brain MRSI assay of tumor pathology at
3T. MRSI data were acquired from a
72-year-old female with a biopsy confirmed oligodendroglioma affecting the left
temporal lobe. To assess 2-HG
concentration, the TE was changed from 32 to 110 ms. All other acquisition parameters were
consistent with those reported above. Metabolite maps for the concentration of
2-HG, tCho, and NAA show marked local disturbance in normal concentration in the
affected area. All concentrations are
shown relative to tCr.