1270
Vagus Nerve Stimulation Promotes Gastric Emptying in Rat Measured by Magnetic Resonance ImagingKun-Han Lu1, Jiayue Cao2, Steven Oleson2, Matthew Ward2,3, Terry Powley4, and Zhongming Liu1,2
1Electrical and Computer Engineering, Purdue University, West lafayette, IN, United States, 2Biomedical Engineering, Purdue University, West lafayette, IN, United States, 3Indiana University School of Medicine, Indianapolis, IN, United States, 4Psychological Sciences, Purdue University, West lafayette, IN, United States
Synopsis
We developed in vivo contrast-enhanced MRI and an image processing pipeline to image and assess the therapeutic effect of cervical vagus nerve stimulation (VNS) on gastric emptying and motility in rat. We found that: (1) VNS significantly promoted the rate of gastric emptying. (2) VNS significantly increased the degree to which the pyloric ring opened, and this outcome was correlated to the rate of gastric emptying. (3) The VNS parameters used in this study did not alter antral motility significantly. The proposed method allows non-invasive and repeated preclinical imaging of gastric physiology and diseases given electroceutical treatments.
Purpose
Impairment of gastrointestinal (GI) functions may lead chronic conditions such as gastroparesis, obesity, or gastroesophageal reflux diseases that are often resistant to pharmacological or dietary treatments. Vagus nerve stimulation (VNS) is an emerging electroceutical technique for remedying gastric disorders, utilizing the vagovagal circuitry that innervates the GI. To evaluate and optimize VNS for GI modulation, we developed a contrast-agent MRI protocol to characterize gastric motility and emptying as the imaging-based readout of gastric physiology.Method
Twenty-one Sprague-Dawley rats (male, 230-330g) were studied according to our protocol approved by the Purdue Animal Care and Use Committee. Animals were trained for seven days to consume voluntarily ~10g of Gd-labeled Dietgel after an 18-hour fast. After eating the Gd-labeled gel on the day of imaging, the animals were anesthetized with 4% Isoflurane for 5 minutes. Of the 21 animals, 10 were imaged under normal physiology and 11 were imaged following the implantation of a bipolar cuff lectrode on the left cervical vagus nerve. Electrical pulses (biphasic square pulses with inter-pulse duration (IPD) = 50ms; pulse amplitude (PA) = 0.6mA; pulse width (PW) = 0.36ms; frequency = 10Hz; 20 seconds on and 40 seconds off) were delivered at the start of image acquisition and lasted throughout the 4-hour experiment, as illustrated in Fig. 1. The animals were scanned in a 7T small-animal MRI system (BioSpec 70/30, Bruker). They first received a subcutaneous (SC) bolus injection of 0.01mg/kg dexmedetomidine solution (0.05mg/ml, Zoetis), followed by a continuous SC infusion of dexmedetomidine at 0.03mg/kg/hr along with 0.3-0.5% isoflurane mixed with oxygen at a flow rate of 500ml/min 15 min after the bolus. Two sets of respiratory-triggered FLASH sequences were used to obtain T1-weighted abdominal images: One for assessing gastric volume with higher spatial resolution and larger spatial coverage (TR/TE = 124/1.4ms; FA = 90°; matrix size = 256 x 256; slice thickness = 1mm; FOV = 60 x 60mm; four averages; and 30 slices) and the other for assessing gastric motility with higher temporal resolution and more targeted spatial coverage of the antrum (TR/TE = 11.8/1.1ms; FA = 25°; matrix size = 128 x 128; slice thickness = 1.5mm; FOV = 60 x 60mm; and 4 slices). The two sequences were interleaved for 4 hours. Gastric volume, as well as antral contraction frequency, amplitude and the velocity of the peristaltic wave, were quantified from the two sets of images using a custom-built image processing pipeline described elsewhere [1]. The degree to which the pyloric ring opened was measured in terms of the cross-sectional area (CSA) of the lumenal content in the pyloric canal from the high spatial resolution scans. One-way ANOVA was used to assess the statistical significance.Results
With a parameter setting informed by electrophysiological findings, the VNS was found to significantly promote gastric emptying (Fig. 2). This effect is attributed to the expansion of the pyloric ring (control vs. VNS: 1.37±0.18 vs. 2.54±0.47 mm2; p < 0.05) (Fig. 3A) without notable changes in antral contraction, in terms of its amplitude (control vs. VNS: 30.58±2.96 vs. 32.46±3.02 % occlusion), velocity (control vs. VNS: 0.67±0.01 vs. 0.67±0.03 mm/s), or frequency (control vs. VNS: 6.34±0.07 vs. 6.42±0.17 cpm) (Fig. 4). The expansion of the pyloric ring was significantly correlated with the rate of gastric emptying, confirming their relationships (Fig. 3B).Conclusion
Here, we reported the use of contrast-enhanced MRI to assess the modulatory effect of VNS on the GI functions in a rodent model. We developed a robust and effective contrast-enhanced MRI technique, along with an animal feeding protocol, to non-invasively monitor gastric emptying and motility. This protocol overcomes the challenge that animals are typically not compliant to test meals, which in turn is a requirement for most human gastric studies. Such experimental protocol was further empowered by advanced image analysis to enable comprehensive assessment of gastric functions and physiology. In summary, the proposed method allows non-invasive and repeated preclinical imaging of gastric physiology and diseases given electroceutical treatments.Acknowledgements
This work was supported in part by NIH SPARC 1OT2TR001965 and Purdue University.References
[1] Lu K-H, Cao J, Oleson S, et al., Contrast enhanced magnetic resonance imaging of gastric emptying and motility in rats. IEEE Transactions on Biomedical Engineering. 2017;64(11):2546-2554.Figures
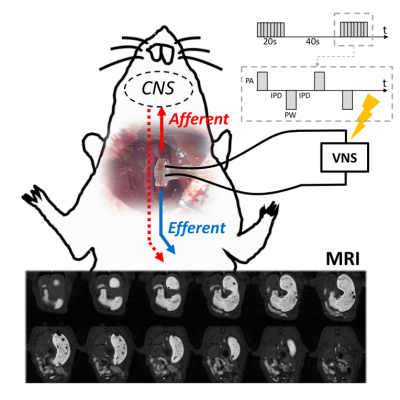
Fig. 1: Based
on the feeding, stimulation and imaging protocol we developed, we collected and
measured gastric emptying profiles and motility indices from high resolution
multi-slice images and fast MR imaging under normal physiology and under VNS.
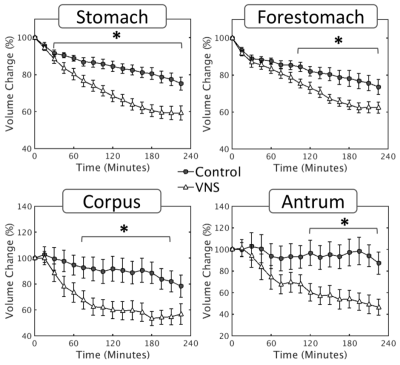
Fig. 2: Vagal
stimulation significantly enhances the rate of stomach emptying at a global
scale, as well as at a compartment-wise scale (forestomach, corpus, antrum). *p<0.05
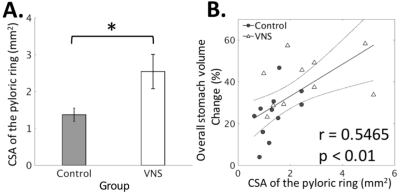
Fig. 3: Quantification
of the degree to which the pyloric ring opens under control vs. VNS condition. A: VNS significantly (*p<0.05)
increases the cross-sectional area (CSA) of the pyloric ring. B: The overall volume change (%; 4-hour
difference) in the stomach is significantly (p<0.01) correlated to
the CSA.
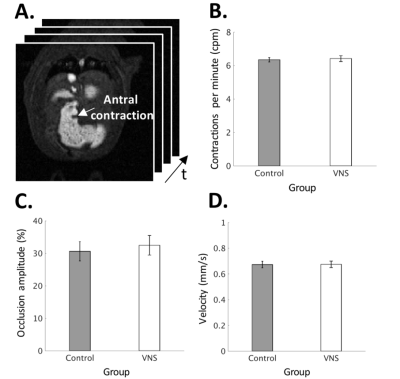
Fig. 4: Quantification
of antral motility under normal vs. VNS condition. A: Fast imaging of the antrum. B:
Antral contraction frequency. C:
Antral contraction amplitude. D: Velocity
of peristaltic wave.