1269
Non-invasive T1 mapping of the vitreous humour can detect central retinal vein occlusion and may discriminate between other forms of retinal ischaemia1Neuroscience, Brighton and Sussex Medical School, Brighton, United Kingdom, 2Sussex Eye Hospital, Brighton and Sussex University Hospital, Brighton, United Kingdom
Synopsis
In this work we demonstrate that careful T1 mapping of the vitreous humour can identify disease in the retina. We studied a cohort of patients with central vein retinal occlusion (a type of retinal ischaemia) and show that significant decreases in T1 of the vitreous humour are observed compared to healthy control eyes. We speculate that the decreases may be the result of increased pO2 that could arise when the oxygen demand of the retina is reduced as a consequence of damage. We show preliminary data from patients with proliferative diabetic retinopathy and ocular ischaemic syndrome that suggest that it may be possible to discriminate different forms of retinal ischaemia completely non-invasively with MRI.
Aim
To assess the viability of MRI as a non-invasive tool to identify (and perhaps discriminate between) diseases of the retina in a cohort of patients that were about to undergo eye surgery.Introduction
The vitreous humour is the clear gel that fills the eye ball between the retina and the lens. Recent research has demonstrated that it has a role in the pathogenesis of a number of conditions such as central retinal vein occlusion (CRVO). Reduced retinal blood supply in conditions such as CRVO is concomitant with a decrease in oxygen supply that triggers a cascade of damage to the retina that may result in severe visual loss. Direct MRI of the retina itself is particularly difficult: it is <0.5 mm thick and suffers from significant movement artefact. However, retinal health can be inferred by probing the vitreous humour that fills the bulk of the eye. The vitreous is in contact with the retina and has the advantage of being largely homogeneous and MR measurements are insensitive to small-scale movement. The partial pressure of oxygen (pO2) can be estimated with MRI using T1 mapping since molecular oxygen is paramagnetic and the longitudinal relaxation rate (1/T1) of the vitreous humour is proportional to pO21-3. In this work we use MR pO2 mapping in the eye to assess the pO2 in a cohort of patients with CRVO to demonstrate that MRI offers a non-invasive approach to detecting abnormalities in the vitreous. We also took preliminary measures of pO2 in patients with proliferative diabetic retinopathy (PDR) and ocular ischaemic syndrome (OIS) to determine whether it may be possible to discriminate between sub-types of retinal ischaemia.
Materials and methods
Scanning protocol: Imaging was performed on a Siemens Avanto 1.5 T scanner (Erlangen, Germany). T1 mapping was performed using an inversion recovery (IR)-trueFISP sequence with 17 inversion times in the range TI=0.7s–30s. A single slice was positioned through the centre of both eyes in the axial oblique plane. Other trueFISP parameters were: TR=(20+TI) s, TE = 1.52 ms, FA = 80°, matrix = 256x256, voxel dimensions = 0.9x0.9x4 mm3. The total scan time for T1 measurement was 15 mins. Eye movement was controlled by instructing the participant to fixate using a visual aid1. Fixation was only required for the duration of k-space acquisition for each TI (approx. 1s only).
Data Analysis: T1 mapping involved a pixel-by-pixel three-parameter fit of the signal intensity S (at each TI) to the equation S(TI) = A + Bexp{-TI/T1}; A and B are parameters that account for inversion pulse flip angle, equilibrium signal intensity and TR. Since flip angle is included, the technique is resilient to B1 errors.
Participants: 8 patients (6 with CVRO, 1 with PDR and 1 with OIS). Age (years) mean=65; range 53-85. Exclusion criteria included bilateral eye disease. In this way, we could use the contralateral eye as a healthy control.
Results and discussion
There is a highly significant (p=0.001) decrease in mean T1 value in the vitreous humour of patients with CRVO compared to T1 measured in healthy control eyes (Figure 1; mean T1 (CRVO)=4.25s, (control)=4.52s). Of note, all CRVO patients exhibited reduced T1 in the vitreous of the diseased eye compared to their healthy eye; none opposed this trend (Figure 2). This finding could be suggestive of increased pO2 as a result of retinal damage. This is particularly intriguing since hypoxic conditions have previously been associated with acute pathology in the retina4. However, it is important to note that these decreased oxygen levels were measured at surgery while hypoxia was suspected to be present as a result of active disease. A possible explanation for increased pO2 in the present study is that when the retina has already been damaged, and it no longer forms an effective oxygen sink. In healthy eyes, the vitreous has a pO2 of approximately 10 mmHg, much less than atmospheric pO2 (130 mmHg) and pO2 in the retinal arterioles (80 mmHg). When the retina is compromised, oxygen diffusing into the eye through the sclera and/or from plasma would lead to an increase in vitreous pO2, as seen here. Exploratory measures of T1 in OIS did not show a reduction compared to measures in control vitreous, while in PDR, a T1 reduction was seen. This may be indicative of the differing levels of retinal ischaemia that characterize these conditions, although we are cautious about drawing conclusions from single-subject measures.
Conclusions
We have demonstrated that T1 in the vitreous humour is significantly and consistently reduced in patients with CRVO. Non-invasive T1 mapping may also permit the discrimination of different forms of retinal ischaemia.Acknowledgements
No acknowledgement found.References
1. Dowell NG, Hughes EH, Simpson ARH, Tofts P. Determination of T1-dependence on oxygenation in the eye using a simple phantom model. Abstract presented at: Proceedings of the International Society of Magnetic Resonance in Medicine(ISMRM); May 5–11, 2012; Melbourne, Australia.
2. Simpson ARH, Dowell NG, Jackson TL, Tofts PS, Hughes EHH (2013). Measuring the Effect of Pars Plana Vitrectomy on Vitreous Oxygenation Using Magnetic Resonance Imaging IOVS, 54:2028-2034.
3. Muir ER, Zhang Y, Nateras OSE, Peng Q, Duong TQ (2013). Human Vitreous: MR Imaging of Oxygen Partial Pressure. Radiology, 266: 905–911.
4. Williamson TH, Grewal J, Gupta B, Mokete B, Lim M, Fry CH (2009). Measurement of pO2 during vitrectomy for central retinal veinocclusion, a pilot study. Graefes Arch Clin Exp Ophthalmol. 247:1019–1023.
Figures
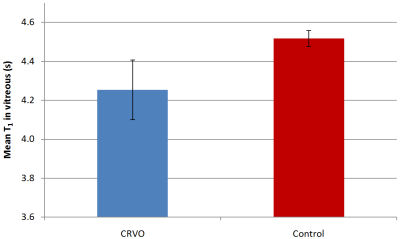
Figure 1 Mean T1 values in the vitreous humour of patients with CRVO retinal ischaemia and contralateral healthy control vitreous. As expected, the variability in T1 is greater in the eyes with CRVO. T1 differences are highly significant p=0.001.
