1267
Magnetic Resonance Imaging Based Assessment of High Fat Diet Induced Mitochondrial Dysfunction Mediated Impaired Lipid Oxidation in Brown Adipose Tissue: Novel Mechanistic InsightsJadegoud Yaligar1, Rengaraj Anantharaj1, Giang Le Thi Thu 1, Ritu Chawla2, Sanjay Kumar Verma1, Venkatesh Gopalan1, Houchun H Hu3, Karthik Mallilankaraman2, and S. Sendhil Velan1
1Laboratory of Molecular Imaging, Singapore Bioimaging Consortium, Singapore, Singapore, 2Department of Physiology, National University of Singapore, Singapore, Singapore, 3Department of Radiology, Nationwide Children’s Hospital, Columbus, OH, United States
Synopsis
Understanding the functional aspects of BAT under obese and overweight conditions is important to improve metabolic dysfunction. Mechanisms that regulate the quality of BAT are influenced by dietary lipids and plays a vital role in fat oxidation. Our imaging and molecular biology data suggests that lipid oxidative capacity of the mitochondria in iBAT is compromised with increased accumulation of lipids. High fat dietary feeding did not affect the mitochondrial content. However, the mitochondrial function was profoundly impaired. The novel finding in this study is the reduction in the activity of complex II in HFD fed rats leading to mitochondrial dysfunction.
Purpose
Investigation of mechanistic interlink between high fat diet induced mitochondrial dysfunction and impaired lipid oxidation in brown adipose tissue.Introduction
Brown adipose tissue (BAT) is involved in energy dissipation by thermogenic process that can modulate fat oxidation under stimulated conditions1-3. Understanding the functional aspects of BAT in response to HFD in obese and overweight conditions is important. Mechanisms that regulate the quality of BAT were influenced by dietary lipids and plays vital role in fat oxidation4. In this study we investigated the mechanistic link between BAT mitochondrial dysfunction and impaired lipid oxidation in response to high fat dietary intervention.Methods
Animal studies were conducted as per the protocols approved by the institutional animal care and use committee (A-star). Male Wistar rats at 7 weeks of age were randomized into two groups and were fed with CD (n=6) and HFD (n=6) diet until 15 weeks of age. Longitudinal MR data was acquired at 7, 11 and 15 weeks of age. Animals were imaged at thermoneutral temperature of 36±0.5 °C and later exposed to acute cold temperature of 18±0.5°C for 1hr. Experiments were performed using a 7T MRI scanner. Spectroscopy experiments were performed in iBAT region TR;4s, TE;13ms, voxel;21mm3, Av;64. After terminal invivo experiments, BAT samples were collected and tissue sections were stained for hematoxylin and eosin staining. Western blot analysis was carried out on lysates from BAT tissues collected. Complex activities were measured from mitochondria isolated from BAT tissues. The mRNA analysis of UCP1 gene was performed on BAT and were normalized to beta actin.Results and Discussion
HFD group rats were insulin resistant by 11 weeks. Figure 1A shows the fat fraction image with a voxel placed in iBAT region. Figures 1B,C,D, E and F show the invivo spectra from iBAT and Figure-1G shows the fat content at thermoneutral and cold exposed condition. At thermoneutral condition the lipid content in iBAT significantly increased for HFD groups. Under cold exposed condition, reduction of fat content for CD fed rats at 7, 11 and 15 weeks was 28.72±1.02%, 16.46%±0.98% and 13.95± 1.13% respectively. For HFD group the reduction of fat at 11w was 12.35±1.08% (P<0.05) and at 15 weeks the fat content did not change significantly in response to acute cold induced BAT activation. Thermogenic function of activated BAT requires the utilization of fatty acids as substrates, leading to uncoupling of protein 1 (UCP1) in mitochondrial membrane. UCP1 (Figure 2A) expression at 11 weeks was increased significantly for HFD group compared to CD group, indicating the metabolic adaptation associated with the compensatory response of mitochondria in response to HFD overload. At 15 weeks, the UCP1 in iBAT of HFD group failed to show this adaptive increase in expression. To further evaluate the oxidizing capacity of iBAT in HFD rats, we studied the expression of CPT1 (Figure 2B,C). In HFD group the CPT1 expression reduced at 15 weeks compared to 11 weeks, indicating the compromised mitochondrial efficiency to oxidize the lipids and is consensus with UCP1 results. We identified the compromised enzyme activity of complex II in iBAT tissues of HFD group. Figures 3A,B,C show Immunoblot analysis and quantification of complex II subunits (SDHA and SDHB) in CD and HFD groups. Complex II enzyme activity (Figure 4A, B) was significantly lower in 11 and 15 weeks old HFD group compared to CD group. Our data suggests that with HFD feeding the mitochondrial content was not affected (Figure 3B,C), however the mitochondrial functionality (Figure 4A,B) was profoundly compromised by HFD feeding. The ROS is essential for iBAT mitochondria and it was significantly decreased in HFD group (Figure-4C). Defective complex II activity reduces the reverse electron transport mediated mitochondrial ROS production which is crucial for BAT mitochondria to activate UCP1. The net effect is down regulation of UCP1 and impaired thermogenesis is mechanistically showed in Figure 5A, B.Conclusions
Our data suggests that lipid oxidative capacity of the mitochondria in iBAT was compromised with increased accumulation of lipids. HFD feeding did not affect the mitochondrial content. However, the mitochondrial functionality was profoundly impaired. Novel finding in our study is reduction in the activity of complex II in HFD group leading to mitochondrial dysfunction. Defective complex II activity reduces the reverse electron transport mediated mitochondrial ROS production at Complex I and Complex III sites which is crucial for BAT mitochondria to activate UCP1 by sulfenylation of UCP1 Cys253 site. Although the UCP1 expression in HFD rats at 15 weeks did not get altered, the activity of UCP1 was down regulated by defective Complex II activity that deprived iBAT from the essential mitochondrial ROS. Our results on defective Complex II mediated decreased UCP1 activity correlates with decreased lipid oxidation observed by MRS.Acknowledgements
No acknowledgement found.References
- Pradhan A, Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders, Nutr Rev. 2007;65(12 Pt 2):S152-6.
- Kristy L, Townsend, et al, Brown Fat Fuel Utilization and Thermogenesis, Trends Endocrinol Metab, 2014 ;25(4):168–177.
- Harms M, et al, Brown and beige fat: development, function and therapeutic potential, Nat Med, 2013; 19(10):1252-63.
- Calderon-Dominguez, M., Fatty acid metabolism and the basis of brown adipose tissue function, Adipocyte, 2016; 5: 98-118.
Figures
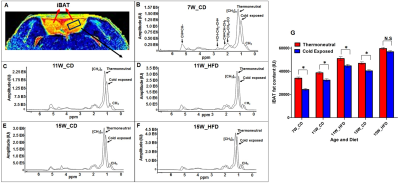
Figure 1A, B, C, D, E. (A) The fat fraction
image with a voxel of interest placed in iBAT region. In vivo spectra from iBAT
at thermoneutral and cold exposed condition of rats fed with CD and HF diets at
(B) 7 weeks, (C, D) 11 weeks and (E, F) 15 weeks. (G) Fat content from iBAT of
CD and HFD fed animals under thermoneutral and acute cold exposure conditions.
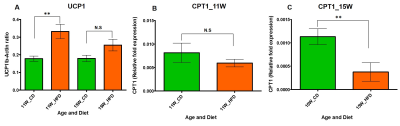
Figure 2 A, B, C. (A) The UCP1 expression
from iBAT of 11 and 15 week old rats fed with CD and HFD. (B) The CPT1
expression from iBAT of 11 week and (C) 15 week old rats fed with CD and HFD.
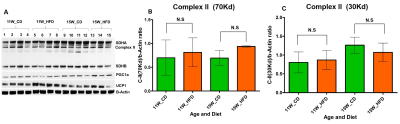
Figure 3 A, B, C. (A) Immunoblot analysis
and quantification of complex II subunits (B) 70 Kd: SDHA (C) 30Kd: SDHB from
iBAT of CD and HFD fed rats.
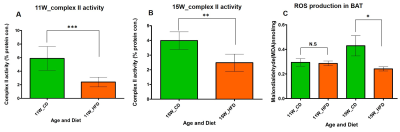
Figure 4 A, B, C. Mitochondrial enzymatic
activity of complex II from iBAT at (A) 11 weeks and (B) 15 weeks old HFD rats.
(C) The lipid peroxidation analysis (measure of reactive oxygen species) from
iBAT of 11 and 15 weeks old rats fed with CD and HFD.
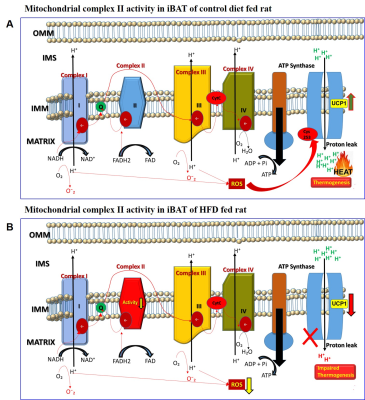
Figure 5 A, B. Schematic diagram
showing the activity of mitochondrial complex II in iBAT of (A) control diet
and (B) high fat diet fed rats. Reduction in the activity of complex II was
observed in HFD group leading to mitochondrial dysfunction. The defective
complex II activity reduces the reverse electron transport mediated
mitochondrial ROS production which is crucial for BAT mitochondria to activate
UCP1. The net effect is down regulation of UCP1 and impaired thermogenesis.