1254
MR-Based Respiratory and Cardiac Motion Corrected PET/MR1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Nuclear Medicine, European University of Brittany, Brest, France, 4British Heart Foundation Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, United Kingdom
Synopsis
A major advantage of hybrid PET/MR systems is the radiation-free high spatial and temporal resolution of MR imaging that can be used to estimate cardio-respiratory motion present during PET data acquisition. This information can be incorporated into reconstruction algorithms to correct for motion in the PET data to reduce blurring and increase target-to-background ratios (TBR) of PET hotspots. This may be of particular importance in cardiac imaging where the heart is in constant motion. In this work, we demonstrate a method for cardio-respiratory motion correction and evaluate the effect on TBR in the myocardium in a cohort of patients.
Purpose
Hybrid PET/MR has significant potential in cardiac applications1 where both functional changes and the pattern of injury can be determined by cine- and late gadolinium enhancement (LGE)-MRI and the activity of disease can be measured by PET. Recently, the potential for PET/MR to evaluate active cardiac sarcoidosis by identifying inflammation in the myocardium using 18F-fluorodeoxygluocose (18F-FDG)-PET and LGE- MR has been demonstrated2. Substantial cardio-respiratory motion during PET data acquisition can cause both image blurring and diminution of target-to-background ratios (TBR), potentially reducing sensitivity for detection of disease. In this work we utilize 3D temporally resolved MRI to estimate respiratory and cardiac motion and apply this to correct for motion in double-gated PET data in a cohort of patients.Methods
Four patients with biopsy-proven extra-cardiac sarcoidosis and suspected cardiac involvement underwent hybrid PET/MR (Biograph mMR, Siemens) to test the feasibility of MR-based motion correction of the PET data. 370 MBq (10 mCi) of 18F-FDG was administered. List-mode PET data acquisition began after 30 minutes and lasted for 60 minutes.
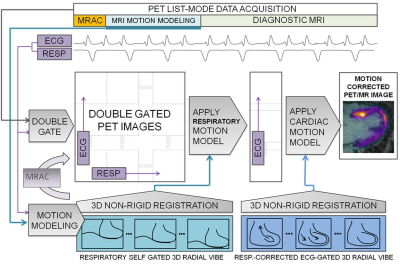
Respiratory motion was estimated using a free-breathing 3D golden-angle radial stack-of-stars sequence (based on Siemens WIP-793 3). A whole-body coronal slab with 3x3x3mm-resolution and 1600 spokes was acquired over 6-7min. The amplitude of the center of k-space was used to provide an estimate of the respiratory phase during the acquisition. Spokes were then divided into 4 respiratory frames based on signal amplitude and reconstructed in Matlab using NUFFT algorithms4. Motion vector fields (MVFs) between respiratory frames were then estimated using freely-available non-rigid registration algorithms5.
Cardiac motion was estimated separately using a similar acquisition but with higher resolution of 1.4x1.4x1.4mm and a coronal slab just covering the heart. In addition, contrast-enhancement (infusion of 0.2mmol/kg Multihance, Bracco) provided additional image-contrast between the blood-pool, coronary vessels and myocardium. Respiratory phase was estimated in the same manner before finding the actual head-to-foot displacement from the images. k-Space data were phase-shifted to correct for the head-to-foot displacement. Corrected k-space data were then sorted into 3 cardiac frames based on recorded ECG trigger timing (0-300, 300-600, 600-inf ms after trigger) before offline reconstruction. MVFs between cardiac frames were then estimated.
To employ PET data acquired during the entire 60 minutes, respiratory phase was estimated from the variation of total PET counts over time, similarly to the method used for MR data. ECG trigger times were then used to double-sort all PET data into a matrix of 4 respiratory and 3 cardiac frames.
Motion correction of PET data employed the reconstruct-transform-average (RTA) approach. Double-gated list-mode PET data were reconstructed offline (e7tools, Siemens) using an iterative algorithm (OP-OSEM, 3 iterations, 21 subsets). Attenuation correction maps6 of the body were transformed using the estimated respiratory MVFs. Finally, RTA was performed in two steps. All cardiac frames were transformed to the end expiration position using corresponding MVFs. Then each respiratory-motion-corrected cardiac frame was transformed to the diastolic position using the cardiac MVFs before averaging all frames (Fig. 1).
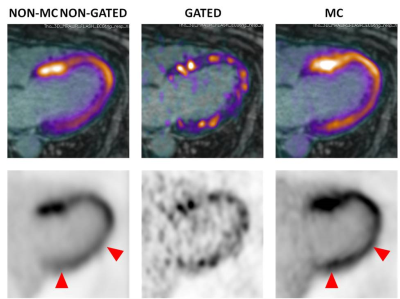
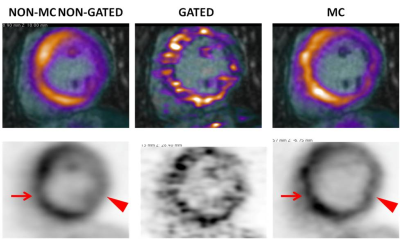
Non-motion corrected non-gated (non-MC non-gated), double respiratory and cardiac gated (gated) and double respiratory and cardiac motion corrected (MC) 18F-FDG-PET images were evaluated qualitatively for image blurring and overall quality. An experienced reader identified regions that were affected by motion by recognizing blurred myocardial features or focal loss of signal. Semi-quantitative analysis compared target-to-background ratio (TBR) in these regions across all three reconstructions using a paired t-test (blood in the left atrium was used to estimate background). Signal-to-noise ratio (SNR) was compared in regions not affected by motion.
Results
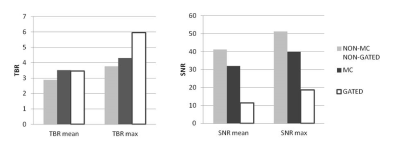
Application of the proposed MR-based motion correction scheme showed qualitative improvement in image quality by reducing blurring of the myocardium, particularly in the lateral wall, and by increasing contrast between the uptake in the myocardium and the background (Fig. 2-3). Semi-quantitative analysis in 8 regions with motion showed a significant increase in TBR values for MC (TBRmean p=0.03) and gated (TBRmean p=0.01) images compared to non-MC non-gated images while there was no difference between MC and gated images (TBRmean, p=0.4). In 9 regions without motion, SNR was slightly lower for MC compared to non-MC non-gated images but was not significant (SNRmean, p=0.11) while SNR was significantly higher compared to gated images for both MC (SNRmean, p=0.01) and non-MC non-gated (SNRmean, p=0.02) images (Fig 4).Discussion
We have demonstrated the feasibility of MR-based correction of cardio-respiratory motion for improved imaging of myocardial PET uptake in 18F-FDG-PET imaging in a cohort of patients, which resulted in increased TBR values whilst preserving SNR. These encouraging results warrant further investigation with and optimization of MR-based motion correction for cardiac PET/MR imaging.Acknowledgements
This work was supported by NIH grant R01 HL071021References
1. Abgral R, Dweck MR, Trivieri MG et al. Clinical Utility of Combined FDG-PET/MR to Assess Myocardial Disease. JACC Cardiovasc Imaging. 2016 Jun 29. pii: S1936-878X. 2. Dweck MR. Abgral R, Trivieri MG et al. Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2017 Jun 9. pii: S1936-878X(17)30450-3 3. Grimm R, Furst S, Dregely I, et al. Self-gated radial MRI for respiratory motion compensation on hybrid PET/MR systems. Med Image Comput Comput Assist Interv 2013;16:17-24. 4. Fessler J et al. Image reconstruction toolbox, University of Michigan (https://web.eecs.umich.edu/~fessler/code/index.html). 5. Buerger C, Schaeffter T, King AP. et al. Hierarchical adaptive local affine registration for fast and robust respiratory motion estimation. Medical Image Analysis, 15:551-564, 2011. 6. Robson PM, Dweck MR, Trivieri MG et al. Coronary Artery PET/MR Imaging: Feasibility, Limitations, and Solutions. JACC Cardiovasc Imaging 2017 10(10):1103-12.Figures