1237
Clinical predictors of serial changes in radiotherapy-induced microbleed burden and their relationship to white matter damage in 133 patients with glioma1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Radiology, University of California San Francisco, San Francisco, CA, United States, 3Neurology, University of California San Francisco, San Francisco, CA, United States, 4Neurological Surgery, University of California San Francisco, San Francisco, CA, United States
Synopsis
In the treatment of adult brain tumors, radiation therapy is associated with long-term effects including vascular injury in the form of cerebral microbleeds (CMBs), and white-matter changes. Ultra high-field MRI was used to detect CMBs, and predictors of CMB development were identified. Changes in individual and total CMB burden were characterized from serial imaging data with white-matter changes. Time since RT, multiple surgeries, tumor type and location were all predictors of development. The total number and volume of CMBs increased over time, while individual CMB size decreased and the surrounding white-matter showed signs of degradation.
Purpose
While radiation therapy (RT) remains a standard practice in the upfront treatment of high-grade gliomas and low-grade gliomas at the time of recurrence, it is often associated with long-term side effects including vascular injury and cognitive decline.1 The former typically manifests as size-varying hemosiderin deposits in the brain called cerebral microbleeds (CMBs), which are capable of being detected with Susceptibility-Weighted Imaging (SWI) as early as 8 months following treatment.2 Usually accompanying CMBs are changes in the integrity of the surrounding white matter (WM) pathways.3 As the median survival of lower grade gliomas can be as high as 10 years with current treatment strategies4, minimizing the deficits incurred through treatment becomes especially important for these patients. Although our prior smaller studies have shown increases in CMB formation over time that were dependent on RT dose5, and that CMBs are not observed in patients treated with chemotherapy alone6, a larger population-based cohort study identifying clinical predictors of CMB development has not been performed. The goal of this study was twofold: 1) to identify significant predictors of CMB development in adults treated with RT for a brain tumor; and 2) to characterize changes in individual CMB size over time compared to alterations in WM structure with serial imaging and using ultra high-field MRI where CMB visualization is greatly enhanced.6-8Methods
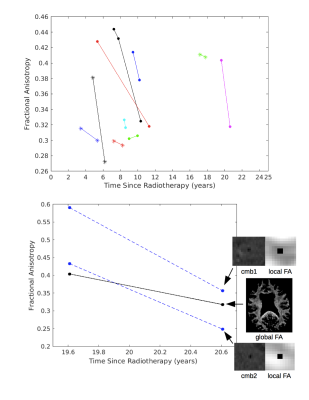
133 patients were scanned on a 7-Tesla GE scanner with a 32 channel receive coil either once or for 2-8 serial scans with varying time between scans. The cohort included patients treated with RT for an adult brain tumor 2 months to 27 years prior, and a subset of nonirradiated control patients. Susceptibility-weighted vascular imaging9,10 was acquired along with T1-weighted images of brain anatomy11, and multi-band, shell, and direction diffusion-tensor imaging12 for a subset of patients. CMBs were detected using an in-house automated CMB detection algorithm13, recently updated to improve specificity and perform CMB volume quantification.14 To identify potential predictors of CMB development, we first performed a series of univariate Poisson regressions. Variables that were associated with CMB development (P < 0.2) were included in the multivariate model, with race, sex, and age at time of RT included as covariates. In the sub-set of patients with serial imaging, mixed-effects models were employed to relate serial changes in CMB number and volume to time since RT using the same covariates. Local and global fractional anisotropy (FA), indicative of WM changes, were also quantified serially and analyzed for trends.Results
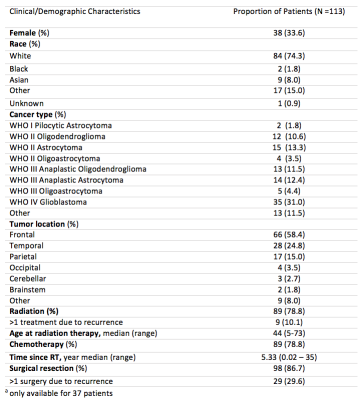
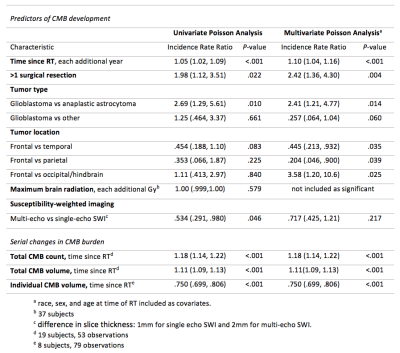
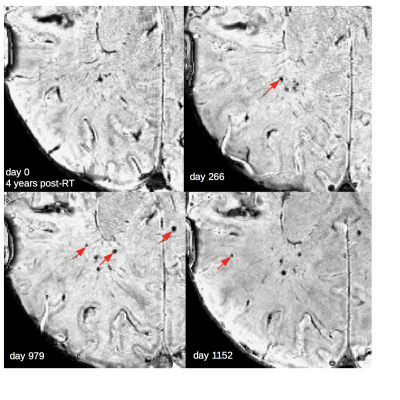
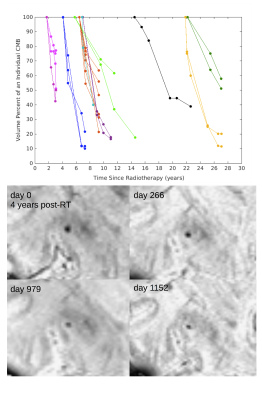
Of the 133 patients, 20 patient datasets were excluded due to poor SWI data quality. Demographics for the remaining 113 patients, including 22 non-irradiated controls, are presented in Figure 1. Four of the 22 non-irradiated controls had evidence of <5 CMBs, a number commonly observed in healthy aging adults.15 In RT-treated patients, the cumulative incident rate for CMB development at 1 year post-RT was 100%. Time since RT, multiple surgeries, tumor type and location were all predictors of CMB development (Fig 2). Maximum brain radiation received was not a predictor of development. The total number and volume of CMBs significantly increased by 18% and 11% per year (rate ratios: 1.18, CI [1.14, 1.22]; 1.11, CI [1.09, 1.13]), respectively, whereas individual CMBs showed significant decreases in volume at a rate of -25% per year (rate ratio: .75, CI [.70, .81]) (Fig. 2 to 4). Some CMBs disappeared all together. Simultaneous to these vascular changes, global and local FA values decreased (Fig. 5). The former showed declines ranging between 1.3% and 21.4% per year (median 6.5%); these measures greatly exceed age-related FA changes reported in older adults.16Discussion & Conclusions
Our preliminary findings suggest that specific brain regions and tumor types influencing the surrounding vasculature may lead to more severe cases of RT-induced vascular injury. Additional surgical interventions after RT may further compromise the integrity of brain vasculature, which can lead to more CMBs. Although our results demonstrate a net increase in total CMB burden with each additional year after RT, individual CMBs decreased in size over time. Occurring over several years, this reduction in CBM volume is a relatively much slower process than their initial rates of formation2. The fact that RT-induced neuronal damage occurs simultaneous with vascular injury provides evidence for long-term disruption in WM integrity due to RT that are independent of normal age-related declines. The outcomes of this work contribute to a more thorough understanding of CMB development and disappearance in this population and support investigations addressing the clinical relevance of CMBs in relationship to cognition in patients with brain tumors.Acknowledgements
The authors would like to acknowledge the assistance of the research staff, MRI technicians, and nurses. A special thanks to Wendy Ma for scheduling patients, and to Angela Jakary, Kim Semien, and Mary Mcpolin, for consenting and scanning. Special thanks are also extended to Quiting Wen and Wei Bian for their contributions to sequence and algorithm development.References
1. Greene-Schloesser, D., Robbins, M. E., Peiffer, A. M., Shaw, E. G., Wheeler, K. T., & Chan, M. D. (2012). Radiation-induced brain injury: A review. Frontiers in Oncology, 2(July), 73.
2. Lupo, J. M., Molinaro, A. M., Essock-Burns, E., Butowski, N., Chang, S. M., Cha, S., & Nelson, S. J. (2016). The effects of anti-angiogenic therapy on the formation of radiation-induced microbleeds in normal brain tissue of patients with glioma. Neuro-Oncology, 18(1), 87–95.
3. Valk, P. E., & Dillon, W. P. (1991). Radiation injury of the brain. American Journal of Neuroradiology, 12(1), 45–62
4. Sanai, N., Chang, S., & Berger, M. S. (2011). Low-grade gliomas in adults. J Neurosurg, 115, 948–65.
5. Wahl M., Anwar M., Hess C. P., Chang S. M., & Lupo J. M. (2017) Relationship between radiation dose and microbleed formation in patients with malignant glioma. Radiat Oncol, 12:126
6. Lupo, J. M., Chuang, C. F., Chang, S. M., Barani, I. J., Jimenez, B., Hess, C. P., & Nelson, S. J. (2012) 7 Tesla Susceptibility-Weighted Imaging to Assess the Effects of Radiation Therapy on Normal Appearing Brain in Patients with Glioma. Int J Radiat Oncol Biol Phys. 82(3): e493-e500.
7. Lupo, J. M., Li, Y., Hess, C. P., & Nelson, S. J. (2011). Advances in ultra-high field MRI for the clinical management of patients with brain tumors. Current Opinion in Neurology, 24(6), 605–15.
8. Bian W., Hess, C. P., Chang, S. M., Nelson, S. J., & Lupo, J. M. (2014). Susceptibility-weighted MR Imaging of Radiation Therapy- induced Cerebral Microbleeds in Patients with Glioma: A Comparison Between 3T and 7T. Neuroradiology, 56:91-6.
9. Bian, W., Banerjee, S., Kelly, D. A. C., Hess, C. P., Larson, P. E. Z., Chang, S. M., Lupo, J. M. (2015). Simultaneous imaging of radiation-induced cerebral microbleeds, arteries and veins, using a multiple gradient echo sequence at 7 Tesla. Journal of Magnetic Resonance Imaging, 42(2), 269–279.
10. Lupo, J. M., Banerjee, S., Hammond, K. E., Kelly, D. A. C., Xu, D., Chang, S. M., Vigneron, D. B., Majumdar, S., & Nelson, S. J. (2009). GRAPPA-based Susceptibility-Weighted Imaging of Normal Volunteers and Patients with Brain Tumor at 7T. Magn Reson Imaging, 27(4):480-88
11. Lupo, J. M., Jakary, A., Hess, C. P., LaFontaine, M., Butowski, N., Clarke, J. L., Chang, S. M., & Larson, P. E. Z. (2017) Anatomical Imaging at 7T for Clinical Assessment of Contrast-Enhancing and T2-hyperintense Lesions in Patients with Gliomas. Minnesota Workshop on High and Ultra-high field Imaging, Minnesota, Minneapolis, 2017.
12. Quiting, W., Kelley, D. A. C., Banerjee, S., Lupo, J. M., Chang, S. M., Xu, D., Hess, C. P., & Nelson, S. J. (2015). Clinically feasible NODDI characterization of glioma using multiband EPI at 7 T. NeuroImage: Clinical, 9(2015):291-99
13. Bian, W., Hess, C. P., Chang, S. M., Nelson, S. J., & Lupo, J. M. (2013). Computer-aided detection of radiation-induced cerebral microbleeds on susceptibility-weighted MR images. NeuroImage: Clinical, 2(1), 282–290.
14. Zou, X., Wei., B, Lafontaine, M., Hess, C.P., Nelson, S.J., Lupo, J.M. (2015). Automated 3-D Segmentation of Radiation-induced Cerebral Microbleeds on Susceptibility Weighted Imaging at 3T and 7T. International Society for Magnetic Resonance in Medicine, Toronto, 2015. Abstract #4393
15. Wiegman, A. F., Meier, I. B., Schupf, N., Manly, J. J., Guzman, V. A., Narkhede, A., Stem, Y., Martinez-Ramirez, S., Viswanathan, A., Luchsinger, J. A., Greenberg, S. M., Mayeux, R., & Brickman, A. M. (2014). Cerebral microbleeds in a multiethnic elderly community: Demographic and clinical correlates. Journal of the Neurological Sciences, 345(0), 125–130.
16. Burzynska, A. Z., Jiao, Y., Knecht, A. M, Fanning, J., Awick, E. A., Chen, T., Gothe, N., Voss, M. W., McAuley, E., & Kramer, A. F. (2017) White matter integrity declined over 6-months, but Dance intervention improved integrity of the fornix in older adults. Frontiers in Aging Neuroscience, 9(59), 1-15.
Figures