1233
Moving Towards A DSC-MRI Consensus: A new single dose option for standardized rCBV.1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
DSC-MRI is the most common approach used for brain tumor perfusion imaging. Consensus is being reached regarding a recommended method for data acquisition, one that requires a contrast agent preload and the application of leakage correction. However, recent simulations suggest that if a low flip angle is used for DSC-MRI data collection then a contrast agent preload might not be necessary. In this study DSC-MRI data collected in 19 patients with brain tumors supports this hypothesis and further suggests that the application of leakage correction as well as a standardization algorithm further improves the results.
INTRODUCTION
Dynamic susceptibility contrast (DSC) MRI is the most commonly used approach to measure brain tumor perfusion. Numerous studies have demonstrated it’s value for predicting tumor grade, overall survival, response to treatment and distinction of tumor from treatment effect. However, full adaption of this approach, for both clinical trials and daily practice, has been impeded by lack of agreement regarding how to best collect and analyze this data1. Yet, there is a growing consensus regarding the benefit of using a preload of contrast agent (CA) and applying post-processing leakage correction methods2-4 for the creation of relative cerebral blood volume (rCBV) maps. The accuracy of this approach has been confirmed by two recent independent studies5,6, which used sophisticated simulations and digital reference objects, and also revealed an additional performance-based option. Specifically, it was shown that DSC-MRI data obtained without a CA preload, with a comparatively lower flip angle (FA) of 30o and a mid-range TE might perform as well as the current standard, which requires a preload of CA. While promising, data is lacking to test this theory. Therefore, the goal of this study was to determine if a single-dose, low-flip-angle DSC-MRI method can provide rCBV information comparable to the standard double dose CA method in patients with brain tumors.METHODS
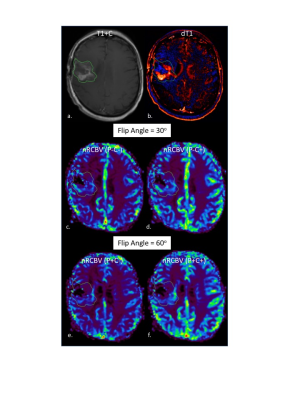
All participants provided informed consent according to IRB policy in this HIPAA-compliant study. Brain tumor patients who were scheduled for an MRI were considered for inclusion in this prospective study. All MRI studies were performed on a 3T MRI. DSC-MRI acquisition was performed twice for each patient, first without a CA preload (P-) using GRE-EPI (FA=30o, TE/TR=30ms/1500ms) and during a bolus injection of CA (0.1mmol/kg Gadavist, Bayer, NJ). Post-contrast T1w (T1+C) images were collected. Next, a 2nd GRE-EPI dataset was collected (FA=60o, TE/TR=30ms/1500ms) during a 2nd bolus dose of CA. This occurs after the CA preload (P+) by virtue of being collected after the T1+C images. All data was post-processed using IB Neuro and IB Delta Suite (Imaging Biometrics LLC, Elm Grove, WI). Normalized (to normal-appearing white matter) rCBV (nRCBV) maps were created with (C+) and without (C-) leakage correction for both DSC-MRI data sets. Similarly, standardized rCBV (sRCBV7) maps (normalization step not required) were also created for both DSC-MRI datasets. Therefore a total of eight different rCBV maps were created for each subject. Delta T1 (dT1) maps were created from the difference of individually standardized pre and post-contrast T1w images using IB Delta Suite. All perfusion maps were co-registered with the T1+C images and mean ROI values extracted from each parameter map. The percent error for each rCBV map was computed in reference to the standard rCBV (acquired with FA=60o and P+/C+) determined for each patient.
RESULTS
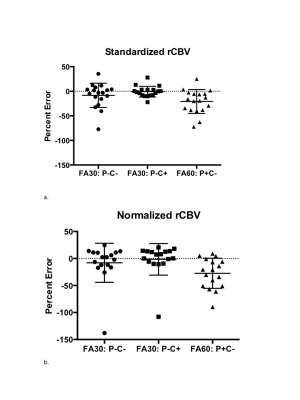
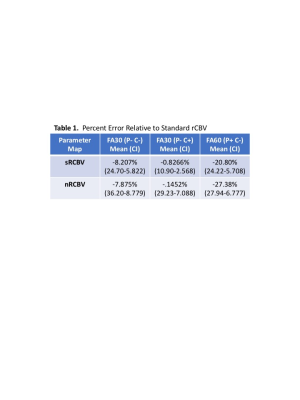
Since January, 2017 21 subjects enrolled in this study. Of these two were excluded from analysis since their enhancing lesion was <1cc. Example T1+C, dT1 and nRCBV maps for each acquisition and post-processing condition are shown in Figure 1. Figure 2 graphs show the percent errors for sRCBV and nRCBV in reference to the accepted standard. The mean percent error and 95% confidence limits for sRCBV and nRCBV are listed in Table 1 (Figure 3). Note that the tumor rCBV obtained with FA=30o and a single dose of contrast agent proved to be most comparable to the standard when leakage correction was used. Moreover, sRCBV shows even better agreement with the standard method, and less variability.DISCUSSION
This is the first study to collect DSC-MRI data in brain tumor patients that enables a direct comparison between single-dose low FA and double-dose standard methods for the creation of rCBV maps. The data support the simulation predictions that comparable rCBV maps can be obtained under single dose low flip angle conditions with leakage correction. While this result is quite promising several study limitations need further consideration. Most study patients were previously treated and some had recurrent tumor. Recurrent tumors are known to have decreased rCBV and less CA leakage in comparison to de novo lesions. Second, all studies were performed at 3T where the consistency across DSC-MRI methods was predicted to be better than at 1.5T.5,6. Therefore, larger studies that include all clinical MRI field strengths and a range of tumor stages must be performed before definitive conclusions can be made regarding the reliability of single dose low FA DSC-MRI methods.CONCLUSION
The results of this study suggest that rCBV data can be reliably determined by using a single dose of CA, a lower FA (recommended to be 30o), a mid-range TE when post-processing leakage correction is applied. This result has substantial implications for accelerating the adoption of DSC-MRI methods for the evaluation of brain tumor patients.Acknowledgements
We would like to thank Cathy Marszalkowski and Steven Jankowski who were instrumental in patient recruitment and MRI data acquisition. We also are grateful for funding support provided by NIH/NCI R01 CA082500 and NIH/NCI U01 CA176110.References
1Paulson, E.S. and K.M. Schmainda, Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology, 2008. 249(2): p. 601-13.
2 Schmainda, K.M., et al., Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol, 2004. 25(9): p. 1524-32.
3Boxerman, J.L., et al., Dynamic Susceptibility Contrast MR Imaging in Glioma: Review of Current Clinical Practice. Magn Reson Imaging Clin N Am, 2016. 24(4): p. 649-670.
4Hu, L.S., et al., Impact of Software Modeling on the Accuracy of Perfusion MRI in Glioma. AJNR Am J Neuroradiol, 2015. 36(12): p. 2242-9.
5 Semmineh, N.B., et al., A Population-Based Digital Reference Object (DRO) for Optimizing Dynamic Susceptibility Contrast (DSC)-MRI Methods for Clinical Trials. Tomography, 2017. 3(1): p. 41-49.
6 Leu, K., J.L. Boxerman, and B.M. Ellingson, Effects of MRI Protocol Parameters, Preload Injection Dose, Fractionation Strategies, and Leakage Correction Algorithms on the Fidelity of Dynamic-Susceptibility Contrast MRI Estimates of Relative Cerebral Blood Volume in Gliomas. AJNR Am J Neuroradiol, 2017. 38(3): p. 478-484.
7 Bedekar, D., T.R. Jensen, and K.M. Schmainda, Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter and intra-patient comparisons. Magn Reson Med, 2010. 64(3): p. 907-913.
Figures