1227
Sub-millimetric 4D Flow MR in small intracerebral aneurysms at 7 Tesla with experimental verification in upscaled 3D printed replica.1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Aerospace Engineering and Mechanics, University of Minnesota, Minneapolis, MN, United States, 3Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 4Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 5Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 6Department Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL, United States, 7St. Anthony Falls Laboratory, University of Minnesota, Minneapolis, MN, United States, 8Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 9Department of Neurology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Asymptomatic intracerebral aneurysms of small size (<7mm) pose a difficult therapeutic challenge: left alone they may stay stable with no consequence or they may grow and/or rupture with devastating subarachnoid hemorrages. Pre-emptive treatment (surgical or endovascular) however carries non-negligible mortality and morbidity and there is no biomarker predicting these relative risk. Flow dynamics inside small aneurysms however could have a critical impact on their evolution. Here we investigate the use of 4D Flow MR to acquire submillimitric flow information in small aneurysms in vivo as well as in 3D printed up-scaled replica of actual aneurysms measured in patients.
Introduction
Small intracerebral aneurysms (IA) affect about 1 in 50 people and are often considered a low risk of rupture. However, the vast majority of IAs that rupture causing potentially devastating subarachnoid hemorrhage (SAH) are < 7 mm in size. Thus, in the presence of asymptomatic, incidentally discovered small IAs, it is unclear which can be safely left alone, and which need to be treated due to risk of future growth/rupture. Preventive treatments (surgical or endovascular) of small IAs carry non-negligible mortality and morbidity. Hence there is an urgent need to identify reliable predictors for the risk of spontaneous growth or rupture in small IAs to guide therapeutic decisions. Some studies suggest that local hemodynamics within IAs play an important role1. Blood velocity and quantitative parameters such as wall shear stress (WSS) can be investigated non-invasively with 4D Flow MR2, and a comparison of these parameters between patients with ruptured and un-ruptured aneurysms can be very useful. Ruptured IAs cannot be scanned in-vivo but, like un-ruptured IAs, can be modeled and 3D printed as upscaled flow phantoms to be scanned with 4D Flow MR at unsurpassed conspicuity. Herein, we investigate the feasibility of measuring detailed blood flow information in small IAs in-vivo (patients with IAs) and in in-vitro (scanning scaled-up 3D printed models of the same IAs), and we compare in-vivo data with corresponding phantom data.Methods
Scans: Four patients presenting with one, and one patient presenting two silent aneurysm(s) were scanned at 7T. Three ECG electrodes were attached to the chest. Time of Flight (TOF) was obtained for lesion localization and vessel mask generation. A cardiac-gated, 4-points 3-directional 4D Flow sequence3 was used to image an axial 3D slab centered on the aneurysm. Relevant parameters: 15 cardiac phases, 2 k-space lines per cardiac phase, voxel size 0.76x0.76x0.8mm3, TR/TE=50.4ms/3.38ms, VENC=0.8m/s, matrix size:288x180x16.
Analysis: DICOM images were processed in Matlab (noise filtering, correction for maxwell term and eddy current, velocity computation); vessel masks were segmented in MimicsTM (based on TOF and/or PC-MRA from 4D Flow data); 3D streamlines and vorticity were calculated and visualized using TecplotTMs ; WSS was computed in matlab and visualized in TecplotTM.
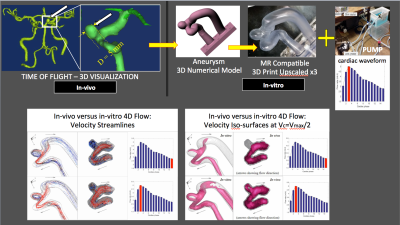
Replica: Based on TOF, PC-MRA or CT-Scan, aneurysms were numerically modelled, upscaled, 3D printed and assembled in an MR-compatible, dynamic flow circuit filled with a fluid chosen for its Reynolds and Womersley numbers4. Time re-scaled patient specific cardiac input functions from in-vivo measurements were fed to a programmable pump. 4D Flow MR data were acquired at 3T with this setting. All technical aspects of this approach, are summarized in Fig.1, and have been reported before4. This process has been completed for 3 small aneurysms scanned in-vivo.
Results
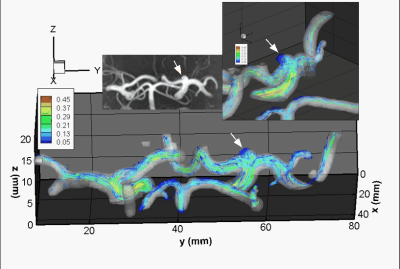
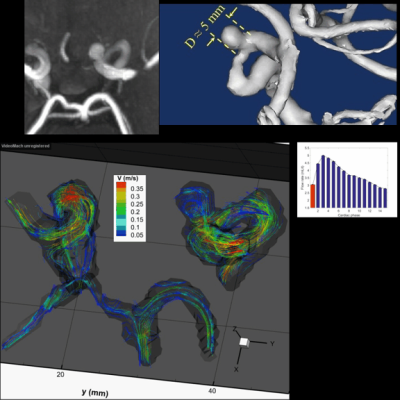
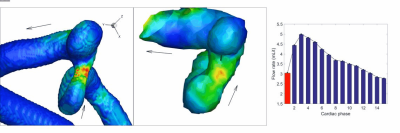
In the patient with two IAs, one was missed (positioning error) while the other proved to be too small (<2mm) to be captured at 0.76x0.76x0.8mm3 resolution, suggesting that 2mm may be the lower limit of our current protocol. In another patient, ECG triggering was corrupted halfway through the scan, degrading image quality. Another subject asked to quit at mid-scan. When successful, however, 4D Flow revealed sharply defined velocity vectors and derived parameters. Typical velocity streamlines are shown for two patients in Figs.2 & 3. One can notice a strong tendency to form circular patterns rotating inside the aneurysm. Further examining the WSS in Fig.4 (same patient as Fig.3), it appears that at some cardiac cycle phases stronger local WSS develop on part of the aneurysm wall, despite a low velocity. 3T measures of 4D Flow in the upscaled 3D printed replica of the same aneurysm (Fig.1) demonstrate fairly similar velocity patterns when compared to in-vivo data.Discussion - Conclusion
We successfully obtained sub-millimetric in-vivo 4D Flow scans in small IAs at 7T. 4D Flow at 3T of aneurysm-specific 3D printed replicas yielded flow patterns comparable to those obtained in-vivo. We believe that the very promising results of this pilot study pave the way for us to study a larger cohort of patients. They also suggest that 3D models of ruptured IAs could provide clinically relevant information in conditions where in-vivo scanning is impossible. Two key targets for improvement were identified. First, ECG issues could be mitigated with acoustic triggering5 and would be less critical in retrospective gating. Second, long 4D Flow acquisition time (~20min) can be shortened with image acceleration techniques (GRAPPA, PEAK)6 which were not used in this pilot phase. Such acceleration, in the case of aneurysms smaller than 1-2mm, may be used to increase spatial resolution rather than reducing scanner time.Acknowledgements
NIH: P41 EB015894, S10 RR029672, U01 EB025144References
1. C. Poelma, P. N. Watton and Y. Ventikos, Transitional flow in aneurysms and the computation of haemodynamic parameters, The journal of the royal society interface, March 2015, http://rsif.royalsocietypublishing.org/
2. M. Markl, S. Schnell, C. Wu, E. Bollache, K. Jarvis, A.J. Barker, J.D. Robinson, C.K. Rigsby, Advanced flow MRI: emerging techniques and applications, Clinical Radiology 71 (2016) 779-795
3 Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 2012;36:1015–1036.
4. O. Amili, D. Schiavazzi , S. Moen, B. Jagadeesan, P.F. Van de Moortele, F. Coletti, Hemodynamics in a giant intracranial aneurysm characterized by in vitro 4D flow MRI, PlosOne (in press)
5. T. Frauenrath, F. Hezel, W. Renz, T. de Geyer d'Orth, M. Dieringer, F. von Knobelsdorff, M. Prothmann, J. Schultz-Menger, T. Niendorf, Acoustic cardiac triggering: a practical solution for synchronization and gating of cardiovascular magnetic resonance at 7 Tesla, Journal of Cardiovascular Magnetic Resonance, 2010, 16;12:67
6. Schnell S, Markl M, Entezari P, et al. k-t GRAPPA accelerated fourdimensional flow MRI in the aorta: effect on scan time, image quality, and quantification of flow and wall shear stress. Magn Reson Med 2014;72(2):522-33.
Figures