1221
Advantages of Dual-Venc 4D flow MRI in the evaluation of cerebral aneurysms1Radiology, Northwestern University, Chicago, IL, United States, 2Neurological Surgery, Northwestern University, Chicago, IL, United States, 3Radiology and Neurological Surgery, Northwestern University, Chicago, IL, United States, 4Biomedical Engineering, Northwestern University, Evanston, IL, United States, 5Radiology, Neurological Surgery and Neurology, Northwestern University, Chicago, IL, United States
Synopsis
We applied and investigated the benefits of using kt-GRAPPA accelerated dual-venc 4D flow MRI in five patients who presented with cerebral aneurysm between 4 and 12mm (smallest and largest dimension). Velocity values were systematically compared with the high-venc-only part of the same acquisition using correlation and histogram analysis. Blood flow was visualized with streamlines and quality was visually assessed. Results show that dual-venc 4D flow MRI provides refined information on slow flow and recirculating flow in cerebral aneurysms. Future studies will assess the feasibility of advanced hemodynamic measures obtained from dual-venc 4D flow MRI as predictive imaging biomarkers.
Introduction
Cerebral aneurysms are life threatening neurovascular lesions, with a significant prevalence of 3-6% in the general population1,2. Although the annual rupture rate is fairly low with < 1-2%3, it is associated with high morbidity and mortality. Risk stratification is currently based on empirical parameters (e.g. patient age, aneurysm size, morphology, and location), or systemic risk factors (hypertension, smoking/alcohol abuse etc.)4,5, but provide an incomplete assessment of this complex disease. In addition, previous studies showed that aneurysm morphology, flow characteristics, and vessel wall properties can be substantially different for individual patients6. Hemodynamic parameters such as inflow jet patterns and wall shear stress have been investigated in previous studies and constitute potential new biomarkers for risk stratification and treatment planning. However, blood flow velocities within aneurysms can differ by an order of magnitude (high inflow jets with 150cm/s vs. slow flow recirculation with 10cm/s versus). The detailed evaluation of complex neurovascular flow occurring inside an aneurysm is thus challenging as it requires the measurement of both slow and fast blood flow velocities within one acquisition. The acquisition of cerebral aneurysmal hemodynamics with a single-venc 4D flow MRI acquisition is thus difficult and would result in either substantial velocity aliasing for high inflow into the aneurysm or high noise levels for low flow velocities in areas with vortex/helix flow. The aim of this study was to perform dual-venc 4D Flow-MRI7 with inherently increased velocity dynamic range in cerebral aneurysm patients and assess the ability to capture slow flow regions as well as circulating flow areas.Methods
A fully integrated dual-venc sequence7 with a shared reference scan (7-point encoding) was used in 5 cerebral aneurysm patients (Table 1) on a 3T Skyra scanner (MAGNETOM, Siemens, Germany). kt-GRAPPA acceleration with R=5 was applied8. A combined dual-venc data set was reconstructed using the high-venc acquisition for complete anti-aliasing of the low-venc data while maintaining a favorable velocity to noise ratio (VNR) of the low-venc data7. Additionally, the high-venc data set from the same measurement (single-venc) was processed for comparison purposes. All 4D flow data was corrected for phase offsets and noise (independently for high-venc and dual-venc), and a phase-contrast angiogram (PC-MRA) was calculated. Based on the PC-MRA, the intracranial vessels and aneurysms were manually segmented (MIMICS, Materialize, Belgium). Segmentations and flow data were loaded into commercial software (ENSIGHT, CEI, USA) for 3D visualization of blood flow through the intracranial vessels and aneurysms. Single-venc (high-venc) and dual-venc scans from the same acquisitions were compared by using velocity histograms and streamline visualization.Results
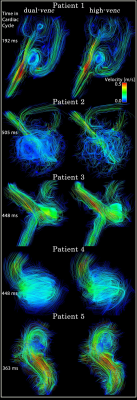
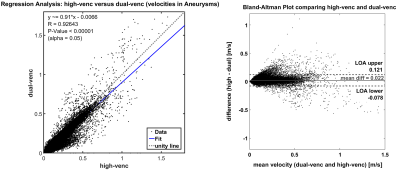
Dual-venc and standard 2D PC-MRI data were successfully acquired (Figure 1) in all 5 patients. Figure 1 shows a comparison of dual-venc vs. single-venc 3D streamlines for visualizing blood flow through the aneurysms. Especially in slow flow regions, dual-venc streamlines were less noisy and more collinear, resulting in an improved depiction of flow patterns (increased streamline density, reduced noise along individual traces) compared to the single-venc acquisition. Figure 2 shows histogram of blood flow velocities occurring in the aneurysms (all voxels in 3D segmentation + time) for dual-venc and single-venc 4D flow MRI, showing a shift to more voxels with slower velocities in the dual-venc scan. Correlation between high-venc and low-venc velocities within the aneurysm demonstrate excellent agreement (R= 0.93, p<0.00001), though from the Bland-Altman plots it can be seen that the higher the velocity value the larger the difference between the measurements (Figure 3).Discussion
Dual-venc 4D flow characterization of hemodynamics in cerebral aneurysms demonstrated less noisy streamlines and more sensitivity to low velocities within the aneurysms, providing a more complete evaluation of the occurring velocities of individual aneurysms. Due to its ability to detect subtle hemodynamic changes for improved aneurysm differentiation, dual-venc 4D flow MRI may have the potential to improve risk stratification by associating Wall Shear Stress differences and intra-aneurysmal 3D velocity distribution with risk of rupture. This 7-point encoded dual-venc 4D flow MRI implementation is a 1.75x longer acquisition than standard single-venc, when acquired without advanced acceleration methods. We acquired this data with k-t GRAPPA to overcome the lengthy acquisition time and could show its advantage over a single-venc acquisition.Conclusion
When applied to patients with cerebral aneurysms, dual-venc demonstrated higher sensitivity to slower velocity values and provided less noisy data. Future longitudinal studies and correlation with aneurysm risk factors or aneurysm growth/rupture are required to evaluate the utility of hemodynamic markers for improved risk assessment and therapy planning.Acknowledgements
Grant support by AHA Scientist Development Grant 16SDG30420005 and NIH R01HL115828References
1. Chen PR, Frerichs K, Spetzler R. Natural history and generalmanagement of unruptured intracranial aneurysms. NeurosurgFocus 2004;17:E1.
2. King JT Jr. Epidemiology of aneurysmal subarachnoid hemorrhage.Neuroimaging Clin N Am 1997;7:659–668.
3. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk ofrupture of intracranial aneurysms: a systematic review. Stroke1998;29:251–256.
4. Rahman M, Smietana J, Hauck E, et al. Size ratio correlates withintracranial aneurysm rupture status: a prospective study.Stroke 2010;41:916–920.
5. Wardlaw JM, White PM. The detection and management ofunruptured intracranial aneurysms. Brain 2000;123(Pt 2):205–221.
6. Schnell S, Ansari SA, Vakil P, Wasielewski M, Carr ML, Hurley MC, Bendok BR, Batjer H, Carroll TJ, Carr J, and Markl M, Three-dimensional hemodynamics in intracranial aneurysms: influence of size and morphology. J Magn Reson Imaging, 2014. 39(1): p. 120-31.
7. Schnell, S., S.A. Ansari, C. Wu, J. Garcia, I.G. Murphy, O.A. Rahman, A.A. Rahsepar, M. Aristova, J.D. Collins, J.C. Carr, and M. Markl, Accelerated dual-venc 4D flow MRI for neurovascular applications. J Magn Reson Imaging, 2017
(8) Jung B, Ullmann P, Honal M, Bauer S, Hennig J, Markl M. Parallel MRI with extended and averaged GRAPPA kernels (PEAK-GRAPPA): optimized spatiotemporal dynamic imaging. Journal of magnetic resonance imaging : JMRI 2008;28(5):1226-1232; 10.1002/jmri.21561.
Figures