1217
Physical Exercise Increased Involvement of Motor Networks as compensatory mechanism under challenging cognitive task1Departments of Psychiatry & Neuroscience, Yale University, New Haven, CT, United States, 2Olin Neuropsychiatry Research Center, Hartford Hospital/Institute of Living, Hartford, CT, United States, 3Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 4Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT, United States
Synopsis
Neuroimaging studies show reorganization of neural resources in older adults may compensate for cognitive decline. To effectively evaluate neural compensation, we proposed a data-driven independent component analysis method, and tested the measure through a longitudinal study. Twenty-six healthy older adults participated in a 6-week physical exercise program. Gait speed, cognitive function, and fMRI during a challenging memory task were measured before and after the program. Results showed a positive correlation between the compensatory ability measure and gait speed at baseline. Physical exercise improved gait speed, cognition, and compensatory ability through increased involvement of motor-related networks in conducting the cognitive task.
Introduction
Methods
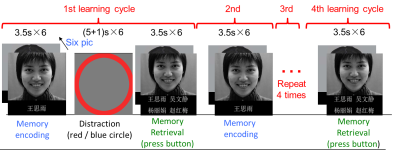
Twenty-six physically and cognitively healthy older adults (mean age (SD) =74.2 (5.7) years) were recruited in this study. Seventeen of them completed a 6-week computer-guided exercise dance program. Gait speed (a measure of physical activity level), cognitive function, and functional magnetic resonance imaging (fMRI) using a very challenging memory task (mean accuracy at 33%) (Fig.1) were measured before and after the exercise program. Gait speed was assessed using the six-minute walking test (6MWT). Cognitive function was evaluated using a neuropsychology battery including Rey Auditory Verbal Learning Test (RAVLT), Logical memory subtest of the Wechsler Memory test (LMT), WAIS-III Digit-Symbol Substitution Modality Test (DSST), WAIS-III Digit span, Trail Making Test (Trails A and Trails B), Stroop Color and Word Test, and Benton visual retention test (BVRT). We used different versions of cognitive tests before and after the 6-week exercise to avoid learning confounds. FMRI data were preprocessed using CONN Toolbox 1 with the standard preprocessing pipeline including slice timing, realignment, registration to structural images, normalization to standard space, and smoothing (6mm kernel). Spatial independent component analysis (ICA) on the memory task-related fMRI data using GIFT toolbox (http://mialab.mrn.org/software/gift/) identified 15 functional network components rather than noise. We then extracted the time course within each component for each subject. Components with a time course significantly correlated (p<0.001) with task design were considered as “activated” by the task, and these were counted as the number of activated networks by the task for each subject. Visual, attentional, and left executive networks were identified as core networks (commonly found in 77.3-97.3% of subjects). Other networks activated by the task were regarded as compensatory. Therefore, we defined the number of activated networks controlled for the volume of core networks as a measure of neural compensatory ability. We examined the relationship between compensatory ability and gait speed at baseline. For each network, the ratio of occurrence of “activated networks” divided by number of all scans was defined as “activated ratio.” Activated ratio changes for each network after exercise were used to examine the network reallocation effect. We applied paired t test to compare changes in cognitive function and gait speed. Significant levels for the t-tests were set as p<0.05 before multiple comparison correction.Results
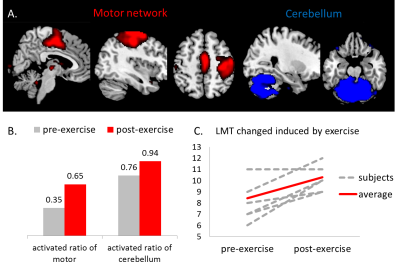
At baseline, the number of activated networks significantly correlated with 6MWT (r=0.66, p=0.015) (Fig.2), and marginally correlated to Digit Span test after multiple comparison correction (r=0.528, p=0.035) (Fig.3a). Only subjects whose accuracy rate was above chance (>25%) were included here. Testing after the exercise program showed significantly improved memory performance in LMT (p=0.001) and RAVLT (p=0.005) and increased gait speed in 6MWT (p=0.03). Among all identified function networks, only the motor and cerebellum networks (Fig.3A) showed increased activated ratio by 30% and 18% respectively (Fig.3B), suggesting a higher involvement of the motor network during memory task performance. When we identified subjects who activated the motor network only following exercise (n=7), we found that all of these subjects showed an increase in LMT (Fig.3C) except for one who showed no change.Discussion
In this study, we proposed a new data-driven measure for neural compensation ability using a highly demanding cognitive task and a brain network-level-based method. To our knowledge, this represents the first report of a direct impact of physical exercise on neural compensation in older adults. A robust neural compensatory mechanism has been considered as part of cognitive reserve. Results indicate that physical exercise could increase the involvement of motor networks as a compensatory mechanism under cognitively challenging task. Interestingly, subjects who gained ability to activate motor system “compensatorily” post- exercise also showed improved logical memory function. We concluded that physical exercise may benefit cognition and motor function, as well as cognitive reserve in older adults.Acknowledgements
No acknowledgement found.References
1. Whitfield-Gabrieli, S. and A. Nieto-Castanon (2012). "Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks." Brain connectivity 2(3): 125-141.Figures