1214
Longitudinal alterations of resting-state functional connectivity in Alzheimer’s disease in a tauopathy mouse model1ICube, University of Strasbourg, CNRS, Strasbourg, France, 2Faculty of Biology, University of Freiburg, Strasbourg, France, 3Dept. of Radiology, Medical Physics, University Medical Center Freiburg, Freiburg im Breisgau, Germany, 4INCI University of Strasbourg, Strasbourg, France, 5LNCA, University of Strasbourg, Strasbourg, France, 6Centres Mémoire de Ressources et de Recherche, CHU de Strasbourg, Services Neurologiques et Gériatriques, Strasbourg, France, 7Département de Biophysique et Médecine Nucléaire, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Synopsis
Alzheimer’s disease is the most widespread cause of dementia and constitutes one of the biggest challenges for society. Among dominant mechanisms of the disease is the abnormal accumulation of the protein tau leading to tauopathy. In this study we explored in vivo the longitudinal evolution of the brain functional connectome, in the Thy-Tau22 mouse, a model of tauopathy. We used resting-state functional MRI in correlation with behavioral analysis to show the remodeling functional circuitry over-time including default mode network and memory networks in transgenic mice.
Introduction and purpose
Alzheimer’s disease (AD) is a neurodegenerative disorder inducing structural, functional and cognitive dysfunctions over time. One of the prior sign of the disease is abnormal functional communication in the brain, occurring even before structural modifications. Resting-state functional MRI (rsfMRI) and diffusion tensor imaging (DTI) are two non-invasive tools allowing a longitudinal investigation of these alterations in vivo. In this context, we combined these two approaches in vivo in a longitudinal study to follow-up and characterize the Thy-Tau22 transgenic mouse model of tauopathy that develops overtime classical AD hallmarks1,2. We investigated the brain connectome in mice from early onset to late stage of the disease, in correlation with learning and memory tests.Methods
Animals:
Thy-tau22 mice present the first neurofibrillary tangles in hippocampus2 at 4 months and a cognitive decline at 8 months1. According to this timeline, two groups of wild-type (WT) C57Bl6/J mice (n=13) and Thy-Tau22 (n=16) mice were characterized at 4, 8, and 12 months via rsfMRI, DTI and behavioral tests.
Behavioral test:
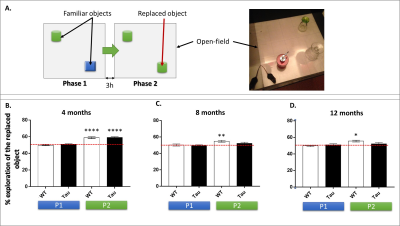
At 4, 8 and 12 months, cognitive abilities of the mice were tested in an object exploration paradigm that evaluates the spontaneous tendency of mice to preferentially explore a replaced object in their environment (object in-place). This test is generally related to hippocampal formation functions3. Behavioral data were analyzed using one sample t-test.
MRI experiments and data analysis:
One week after behavioral evaluation, brain MRI
was carried-out in the two animal groups using a 7T small bore animal scanner (BioSpec 70/30, Bruker, Germany) and a mouse head adapted room temperature surface
coil. RsfMRI data were acquired using single shot GE-EPI (TE/TR = 15ms/2000ms),
27 axial slices of 0.4mm thickness (FOV: 2.12x2 cm², resolution: 0.14×0.22mm2,
500 volumes). DTI data were acquired using a DTI-EPI sequence, with 30
directions, 6 b-values and a 0.1x0.1x0.5mm3 resolution. Scans were realised
under medetomidine (MD) sedation (subcutaneous bolus of 0.6mg MD/kg body weight
followed by s.c. infusion of 0.3mg MD/kg-BW/hour) for rsfMRI acquisitions, and
under isoflurane for DTI acquisitions. Data
pre-processing was performed using SPM 8 for motion correction, coregistration
to a study based template and spatial normalization to the Allen Mouse Brain
Atlas as previously described4. For rsfMRI data, a smoothing of 2 voxels, a
frequency filtering (0.01 to 0.1Hz) and a removal of ventricles signal were
applied before extracting regions of Interest (ROIs). These ROIs were then used
in a seed-based correlation analysis.
To perform structural analysis, a global
tracking5 of DTI data
in association with statistical analysis of DTI maps were realized.
Results and Discussion
Starting from 8 months of age, Thy-Tau22 mice underperformed in behavioral tests compared to controls (Fig 1), indicating a progressive loss of memory in transgenic mice that may be related to the accumulation of neurofibrillary tangles throughout the brain2 .
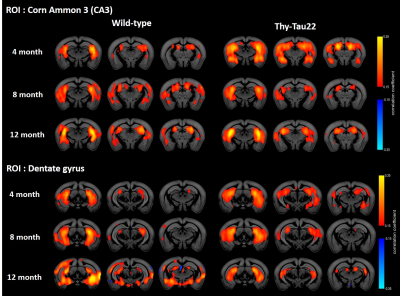
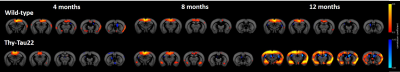
However rsfMRI mapping of brain functional connectivity (FC) showed abnormal modifications in BOLD signal of specific networks at already 4 months of age in Thy-Tau22 mice. Notably, hippocampus-amygdala network (Fig 2) as well as hippocampus-somatosensory connectivity seemed to be strongly increased in transgenic mice compared to WT mice at this age (Fig 2). In coherence with behavioral results (Fig 1), analysis of FC in Thy-Tau mice at 8 and 12 months showed a loss of the hyperconnectivity of the CA3 network and a decrease of dentate gyrus FC, compared to WT mice (Fig 2). Finally, strong remodeling of the retrosplenial cortex FC - associated to the Default Mode Network (DMN) in mice - was found in Thy-Tau22 mice. DMN shows progressive increase of its FC with the rest of the brain from 8 months of age, displaying significantly stronger FC patterns at 12 months (Fig 3) when compared to the DMN of control mice.
Overall, these results suggest that the 4 months overactive resting state hippocampal network potentially underlie modifications related to early tau accumulation in hippocampus. The progressive decline of hippocampal FC at 8 months in Thy-Tau22 mice may be related to a shift of the hippocampus processing toward another core processing hubs in the brain, like the DMN. The increase of the DMN connectivity in transgenic mice starting at 8 months might indicate the overloading of this network. This evolution of the FC in the brain of Thy-Tau22 mice is consistent with the cascading network failure model of Alzheimer’s disease6 that predict networks changes which would be related to both amyloid and tau. To our knowledge the results presented here are new observations in mouse models of tauopathy.
Further analysis of the DTI data acquired in this study and immunohistological investigations will provide more information about responses and adaptations in AD from early to late stage of the disease.
Acknowledgements
No acknowledgement found.References
- Van der Jeugd, A. et al. Progressive age-related cognitive decline in tau mice. J of Alzheimer’s Dis: JAD 37, 777–788 (2013).
- Schindowski, K. et al. Alzheimer’s Disease-Like Tau Neuropathology Leads to Memory Deficits and Loss of Functional Synapses in a Novel Mutated Tau Transgenic Mouse without Any Motor Deficits. The American Journal of Pathology 169, 599–616 (2006).
- Barker, G. R. I. & Warburton, E. C. Object-in-Place Associative Recognition Memory Depends on Glutamate Receptor Neurotransmission Within Two Defined Hippocampal-Cortical Circuits: A Critical Role for AMPA and NMDA Receptors in the Hippocampus, Perirhinal, and Prefrontal Cortices. Cereb Cortex 25, 472–481 (2015).
- Arefin, T. M. et al. Remodeling of Sensorimotor Brain Connectivity in Gpr88-Deficient Mice. Brain Connect 7, 526–540 (2017).
- Harsan, L.-A. et al. Mapping remodeling of thalamocortical projections in the living reeler mouse brain by diffusion tractography. Proceedings of the National Academy of Sciences 110, E1797–E1806 (2013).
- Jones, D. T. et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex (2017). doi:10.1016/j.cortex.2017.09.018
Figures