1211
Optogenetic fMRI dissection of brain-wide vestibular pathways1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
The vestibular system is essential to our sense of balance and spatial orientation. fMRI mapping of the vestibular system has been challenging due to the physical constraints limiting a subject’s ability to perform motion, balance and orientation related tasks within an MRI scanner. At present, our knowledge of the brain-wide cortical and subcortical regions that participate in processing the vestibular sense is scarce. Here, we combine fMRI and optogenetic stimulation of vestibular excitatory neurons and, for the first time, successfully map the multiple brain-wide vestibular pathways.
Purpose
Both basic and clinical research communities have employed fMRI to map local brain functions by measuring neuronal activities throughout the brain in response to specific sensory or cognitive tasks. This approach has been particularly informative in defining sensory and motor regions1, whereby the functions are topographically organized. However, defining vestibular (balance and spatial orientation related) regions and examining their functions via traditional fMRI mapping approaches is technically challenging. Subjects positioned inside a scanner during fMRI experiments are generally unable to perform vestibular tasks such as head and body rotational and/or translation movements. At present, our understanding of the brain-wide cortical and subcortical regions that are involved in processing the vestibular sense is incomplete. Recently, the combined use of optogenetic and fMRI has enabled us to perturb long-range networks through focal, cell-type specific, neural stimulation; and simultaneously monitor the brain-wide neural activities evoked by such optogenetic perturbation2,3. Taking advantage of this capability, we examine the functional pathways of the vestibular system. We aim to map and categorize the downstream targets of the vestibular nucleus (VN) across multi-synaptic pathways spanning the entire brain by optogenetically activating medial VN (MVN) excitatory neurons.Methods
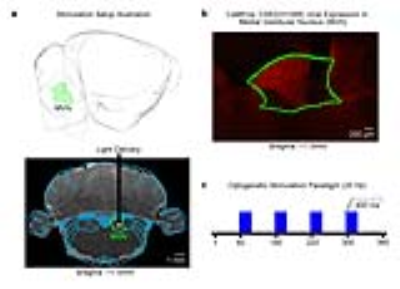
Animal preparation and optogenetic stimulation: 3μl of AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to MVN (-11.5mm posterior to Bregma, +1.5mm medial-lateral right hemisphere, -8.5mm from surface of dura) of adult rats (200-250g, male, SD strain, n=6). Four weeks after injection, an opaque optical fiber cannula (d=450μm) was implanted at the injection site (Figure 1a, b). Blue (473nm) light was presented to animals expressing ChR2 at 20Hz (20% duty cycle, 40mW/mm2) in a block-design paradigm (20s on and 60s off; Figure 1c).
fMRI acquisition and analysis: fMRI data was acquired on 7T Bruker scanner using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 16 contiguous slices with 1mm thickness). Data were preprocessed before standard GLM analysis was applied to identify significant BOLD responses (p<0.01; FDR corrected).
Results
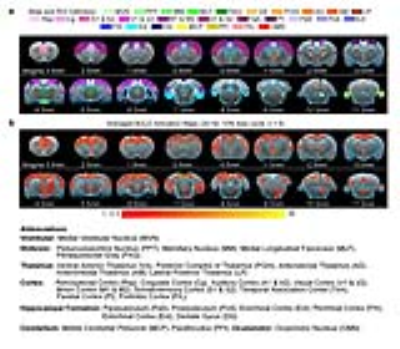
Brain-wide fMRI mapping of downstream signal propagation from MVN: We detected robust large-scale BOLD fMRI activations at numerous cortical, hippocampal formation and subcortical regions (Figure 2). Notable regions that were activated include sensorimotor cortices and their associated thalamus (auditory, visual, somatosensory and motor), high order cortices involved in cognition (cingulate, retrosplenial, temporal association and parietal), and the hippocampal formation involved in spatial navigation (dentate gyrus, entorhinal cortex and subiculum). Furthermore, we found broad activations at the midbrain which has extensive projections to thalamic and hippocampal formation regions (mammillary nucleus and periaqueductal gray). As expected, the oculomotor nucleus, an essential midbrain region mediating the vestibulo-ocular reflex, was also activated.
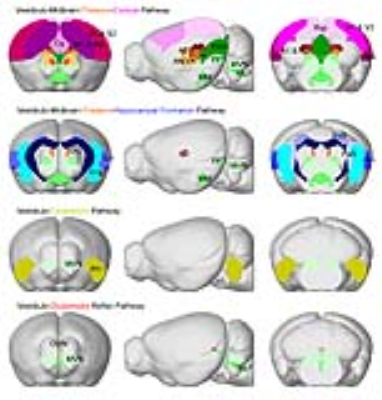
Categorization of distinct brain-wide vestibular functional pathways identified from fMRI activations: We identified four distinct pathways that were activated by the optogenetic excitation of MVN (Figure 3). The pathways include the vestibulo-midbrain-thalamo-cortical, vestibulo-midbrain-thalamo-hippocampal formation and vestibulo-midbrain-hippocampal formation, vestibulo-cerebellum, and vestibulo-oculomotor reflex pathways. The above fMRI visualization demonstrates that evoked neural activity in MVN propagates to multiple brain regions and forms long-range functional pathways in the vestibular system.
Discussion
In this study, we successfully demonstrated for the first time the capability of fMRI in mapping and categorizing the multi-synaptic pathways of the vestibular system by optogenetically stimulating MVN excitatory neurons. From the robust BOLD activations, we categorized the activation of four distinct brain-wide pathways (Figure 3). First, we detected the activations across the vestibulo-midbrain-thalamo-cortical pathway, which includes sensorimotor thalamo-cortical regions, and higher order sensory association cortices. This result confirms the indispensable role of the vestibular system in integrating various sensory inputs4. Second, we observed activations along the vestibulo-midbrain-thalamo-hippocampal formation/vestibulo-midbrain-hippocampal formation pathway. This finding supports the view that the vestibular system has substantial roles in cognitive processes such as spatial memory, navigation and learning as previously reported in several electrophysiological studies5,6. Third and interestingly, we detected activations in the vestibulo-cerebellum pathway. The vestibular-cerebellum pathway is widely postulated to interact with the thalamic, cortical and hippocampal formation regions6,7. Lastly, as expected, we observed the activation of the vestibulo-oculomotor reflex pathway that courses through three synapses such as the medial longitudinal fasciculus, oculomotor nucleus and oculomotor muscle (not covered in our imaging plane)8.Conclusion
The present study demonstrates for the first time the ability of optogenetic fMRI to map brain-wide vestibular pathways. Our findings provide direct experimental evidence for the presence of four multi-synaptic pathways associated with vestibular sensing in the brain. This optogenetic fMRI approach, in conjunction with electrophysiological measurements, offers the exciting possibility to further interrogate the vestibular system and functions in the future.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
- Belliveau, J.W., Kennedy, D.N., Jr., McKinstry, R.C., Buchbinder, B.R., Weisskoff, R.M., Cohen, M.S., Vevea, J.M., Brady, T.J. & Rosen, B.R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254, 716-719 (1991).
- Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
- Leong, A.T., Chan, R.W., Gao, P.P., Chan, Y.S., Tsia, K.K., Yung, W.H. & Wu, E.X. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc Natl Acad Sci U S A 113, E8306-E8315 (2016).
- Vidal, P.P., Cullen, K., Curthoys, I.S., Du Lac, S., Holstein, G., Idoux, E., Lysakowski, A., Peusner, K., Sans, A. & Smith, P. Chapter 28 - The Vestibular System A2 - Paxinos, George. in The Rat Nervous System (Fourth Edition) 805-864 (Academic Press, San Diego, 2015).
- Hitier, M., Besnard, S. & Smith, P.F. Vestibular pathways involved in cognition. Frontiers in integrative neuroscience 8, 59 (2014).
- Cullen, K.E. & Taube, J.S. Our sense of direction: progress, controversies and challenges. Nat Neurosci 20, 1465-1473 (2017).
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807-815 (2013).
- Dieterich, M. & Brandt, T. Vestibulo-ocular reflex. Curr Opin Neurol 8, 83-88 (1995).
Figures