1205
Diffusion-prepared multi-shot bSSFP imaging with gradient stabilizer1Department of Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine IDP, University of California, Los Angeles, Los Angeles, CA, United States, 3Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 4Siemens Healthcare, Los Angeles, CA, United States, 5Department of Radiation Oncology, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
A gradient stabilizer strategy was proposed to solve the k-space magnitude inconsistency problem in multi-shot diffusion-prepared bSSFP imaging. Simulation studies showed that the proposed approach is insensitive to phase errors during the diffusion encoding stage, and has built-in fat-saturation property. Phantom and in-vivo studies verified that adding gradient stabilizers could remove signal loss and artifacts, and provide repeatable artifact-free images. Combined with existing phase correction techniques, the proposed approach provided distortion-free high-quality 2D diffusion-weighted and diffusion tensor images, and has the potential of extending to 3D.
Introduction
Diffusion-prepared imaging approach is gaining popularity due to its flexibility of combining with any types of readout including balanced steady-state free precession(bSSFP), which has the promise of achieving fast 3D multi-shot distortion-free diffusion1. However, shot-to-shot inconsistency is inevitable in multi-shot diffusion-weighted imaging. Even in the brain, phase inconsistency caused by eddy currents and non-rigid pulsatile motion could lead to destructive signal loss and ghosting artifacts that cannot be easily resolved by low-order motion compensation. Various reconstruction methods have been proposed to mitigate the phase variation problem in diffusion-weighted approach2,3. However, in diffusion-prepared approach, the use of spoilers after the -90˚ tip-up pulse induced a shot-to-shot magnitude inconsistency. This magnitude variation is more challenging to be alleviated due to the complementary parts have already been spoiled. In this work, we propose to use a gradient stabilizer approach to convert the magnitude problem back to the well-studied phase problem. In this way, the images can be reconstructed using existing well-developed methods.Methods
Sequence: Figure 1(a) shows the sequence diagram of the proposed gradient-stabilized diffusion-prepared bSSFP sequence (GS-DP-bSSFP, abbreviated as GS-DP. Sequence without GS is abbreviated as DP). Inspired by Alsop4, one 4π dephasing gradient is placed before the -90˚ pulse. Rephrasing and dephasing gradients are played during each readout. As illustrated in Figure 1(b)-(c), those gradients convert the magnitude inconsistency back to the phase inconsistency in diffusion-prepared acquisition.
Simulation: Bloch simulations and Spin Bench simulation(HeartVista, Mountain View) were performed to simulate the effect of adding gradient stabilizers with respect to phase error and off-resonance. In the first simulation, a -π to π phase error was added before the -90˚ pulse to 1000 simulated spins. Signal magnitude of the first echo was plotted. In the second simulation, 440Hz off-resonance(off-resonance of fat at 3T) was studied. Signal magnitude during the echo train with and without GS and with and without off-resonance was compared.
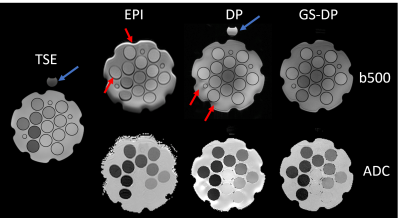
Phantom: A diffusion phantom with a fat tube on top was used to quantify the ADC accuracy of the proposed GS-DP sequence. TSE, diffusion-weighted single-shot EPI(DW-ssEPI), DP, and GS-DP images were acquired on a 3T scanner(Prisma, Siemens) with the following parameters: Resolution=1.0x1.0mm2/1.9x1.9mm2/1.2x1.2mm2/1.2x1.2mm2, TE=85ms/97ms/45ms/45ms, number of shot=16/1/4/4, and TR=3ms for the four sequences mentioned above respectively. B-values of 0, 200 and 500 s/mm2 were acquired in the three diffusion sequences. Fat-saturation was only applied in the DW-ssEPI scan.
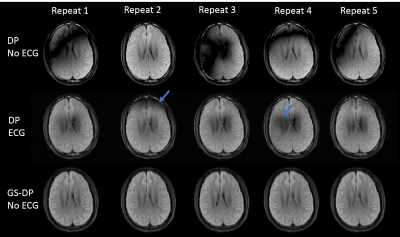
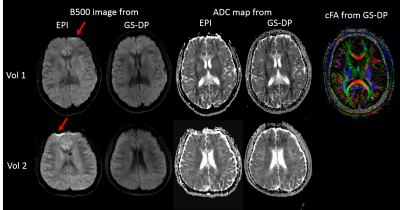
In-vivo study: Five healthy volunteers were recruited to evaluate the in-vivo feasibility. To study the benefits of the proposed approach, DP without ECG gating, DP with ECG gating, and GS-DP without ECG gating were acquired ten times with b=500s/mm2 in a single-shot manner. To evaluate the multi-shot feasibility, DW-ssEPI and 4-shot GS-DP (no ECG) were acquired with b=0, 200 and 500 s/mm2. In one volunteer, 4-shot GS-DP with b=500s/mm2 was acquired along 15 directions for the diffusion tensor reconstruction. All multi-shot GS-DP images were reconstructed using the MUSE technique3.
Results and Discussion
Without gradient stabilizers (Figure 2(a)), the signal magnitude exhibited a strong dependence on the phase error. Adding gradient stabilizers maintained the signal magnitude but with the price of half of signal loss. Figure 2(b) is the off-resonance simulation. At 440Hz off-resonance, the signal magnitude in DP was approximately three times higher than the 0Hz case and had strong oscillation; whereas in GS-DP, the signal is suppressed at 440Hz.
As shown in Figure 3, the proposed GS-DP approach had substantially reduced distortion and susceptibility-related artifacts compared to DW-ssEPI. Susceptibility related artifacts around the tube/phantom boundary were also reduced compared to DP. The fat tube produced a negligible signal in GS-DP although no fat saturation was applied. This is consistent with the simulation result shown in Figure 2(b). For tubes that has diffusivity over 0.5x10-3 mm2/s, the ADC difference between DW-ssEPI and GS-DP was within 4%.
Without GS and ECG gating, the images had severe signal void artifacts (Figure 4). Those magnitude variations were hard to compensate due to the complementary parts have already been spoiled. ECG gating could reduce the artifacts; however, magnitude variations were still observable. Adding the gradient stabilizer provided stable signal magnitude across all repetition which is essential for high-quality multi-shot imaging.
Multi-shot images from two volunteers are shown in Figure 5. Distortion and susceptibility-related artifacts were substantially reduced using the GS-DP approach. For selected ROIs on the grey matter, white matter and CSF, ADC differences between GS-DP and DW-ssEPI were within 5%. High-resolution high quality color-coded fractional anisotropy map was generated using the GS-DP approach.
Conclusion
The proposed GS-DP approach provided high-quality 2D multi-shot diffusion images and has the potential of achieving fast 3D distortion-free diffusion imaging.Acknowledgements
No acknowledgement found.References
1. Nguyen C, Sharif-Afshar A-R, Fan Z, et al. 3D high-resolution diffusion-weighted MRI at 3T: Preliminary application in prostate cancer patients undergoing active surveillance protocol for low-risk prostate cancer. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. March 2015. doi:10.1002/mrm.25609.
2. Jeong E-K, Kim S-E, Parker DL. High-resolution diffusion-weighted 3D MRI, using diffusion-weighted driven-equilibrium (DW-DE) and multishot segmented 3D-SSFP without navigator echoes. Magn Reson Med. 2003;50(4):821-829. doi:10.1002/mrm.10593.
3. Chen N-K, Guidon A, Chang H-C, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage. 2013;72:41-47. doi:10.1016/j.neuroimage.2013.01.038.
4. Alsop DC. Phase insensitive preparation of single-shot RARE: application to diffusion imaging in humans. Magn Reson Med. 1997;38(4):527-533.
Figures